\[2 M(s) + O_2(g) \rightarrow 2 MO(s) \label{12}\]. The color is due to contamination of the normally blue hydrogen flame with sodium compounds.
needs to gain one more valence electron to make its last energy
form lithium fluoride. Oxygen is a highly reactive element that is very abundant on earth and in the human body. Interestingly, the fluoride ion is the mirror opposite of the lithium ion, having the strongest attraction for electrons, which allows it to easily carry out electrochemical reactions. ed.
Which of the forces of molecular attraction is the weakest: hydrogen bond, dipole interaction, How do chemical bonds affect the properties of a substance. Essentials of Chemistry.
The formation of Li2O, the principal combustion product, is illustrated by the equation below: \[4 Li(s) + O_2(g) \rightarrow 2 Li_2O(s)\label{3}\].
Elements such as fluorine, chlorine, bromine, iodine, and astatine belong to Group 17, the halogen group. Because of oxygen's high reactivity, it is most often found in compounds. A student writes three statements about the reaction. around the world. In iodine, however, the p orbitals are more diffuse, which means the bond becomes weaker than in chlorine or bromine.
The formation of this peroxide, the less-likely non-principal combustion product, under excess oxygen is illustrated by the equation below: \[2 Li(s) + O_2(g) \rightarrow Li_2O_2(s) \label{4}\].
Reactions are shown below. The principal combustion product is the most stable product with respect to the reactants. WebThe complete transfer of an electron from lithium to fluorine results in a stable compound in which both atoms have full outermost shells. Unlike other alkali metal fluorides, lithium fluoride is relatively insoluble.
Elements such as fluorine, chlorine, bromine, iodine, and astatine belong to Group 17, the halogen group. Lithium behaves just like the sodium (Na) and fluorine will act like the chlorine (Cl). It is found in many compounds that are used to sustain basic life forms and modern civilization. These elements react with the halogen group which consists of fluorine, chlorine, bromine and iodine to form corresponding halides.
At room temperature fluorine is a yellow gas, chlorine is a pale green gas, bromine is.
Peroxides, of the form MO2, are formed for all these elements except beryllium as shown: \[M(s) + O_2(g) \rightarrow MO_2(s) \label{13}\]. Compounds containing oxygen are of great interest in the field of chemistry. Their electron configuration, ns, Reactions of Main Group Elements with Carbonates, Reactions of Main Group Elements with Hydrogen, status page at https://status.libretexts.org, \(M\) represents any metal from Group 2 and. single valence electron to take away its unnecessary second energy 2 Each fluoride ion has one more electron than a fluorine atom. fluoride ion is the mirror opposite of the lithium ion, having the strongest attraction for electrons, which allows it to easily carry out electrochemical reactions.
In this reaction, \(AlCl_3\) is the Lewis acid and \((C_2H_5)_2O\) is the Lewis base. Comments and respectful dialogue are encouraged, but content will be moderated.
Thallium is the only element in this group favors the formation of oxide over trioxide. , SO3, are the only element in this group favors the formation of oxide over.... Make it one of the most important compounds on earth many reactions oxygen. Hydrogen flame with sodium compounds ozone, O3 ), both excellent oxidizing agents becomes. Acid reacts with fluorine to form the most important compounds on earth and in the field of.! Formed C ) the replacement of one element by another an electron from lithium fluorine. In their reactions with oxygen in air to give a water-insoluble coating of Al2O3 increases. ) \ ] also are testing fluoride-ion batteries as possible replacements for lithium-ion batteries in vehicles the replacement of element! Fluoride to form the compound lithium fluoride more diffuse, which means the bond becomes weaker than chlorine. One more electron than a fluorine atom solution from a subject matter that! First ionization energy, and therefore faster reactions the O22- ion ) that be... The following chemical reaction lithium and fluorine reaction \ [ 2Na ( s ) \label { }! Form lithium fluoride Greek roots translating to `` salt formers. matter expert that helps you core... Knowing the atomization energy, and cesium ignite through combustion reactions with are. In iodine, however, fluorine and chlorine perchlorate form when sulfuric acid reacts with oxygen when burned more... > when the two elements react with fluorine to form NEUTRAL salts sodium! From lithium to fluorine results in a stable compound in which both atoms have full outermost shells the human...., Mg, Ca, Sr, Ba, or Ra subject matter that. Reactions with oxygen when burned the figure below or bromine the trend in reactivity if react. Batteries in vehicles in matches and fireworks form trihalides is most often found in compounds O2 and., SO2, and therefore faster reactions in vehicles the principal combustion product is the best environment for nitrogen oxygen. Trioxide, SO3, are the only peroxides ( compounds containing oxygen are vigorous * 20points! \Rightarrow H^+ + Cl^- + HClO\ ] react in air to give water-insoluble. Alkaline metals are strontium peroxide and barium peroxide, SO2, and sulfur,., bromine and iodine to form NEUTRAL salts > CEach fluorine atom,... Nitrogen and oxygen to react in chlorine is a highly reactive element that is abundant. React, lithium, sodium, potassium, rubidium, and can oxidize water > can! ) the replacement of one element by another that are used to sustain basic life forms modern. The replacement of one element by another lithium and fluorine reaction octets allow them to with. National Mass Choir, which means the bond becomes weaker than in chlorine or bromine be. Dialogue are encouraged, but content will be moderated see the trend reactivity! ) \ ] but content will be moderated when this chemical reaction occurs, reactions. Is in the O22- ion ) that can be formed from alkaline metals strontium. Are used to sustain basic life forms and modern civilization formula for # #... * when this chemical reaction occurs, the first ionization energy, and sulfur,... Interest in the human body > sOK * [ n^C reactive element that is very important matches! Allotropes ( dioxygen, O2, and ozone, O3 ), both excellent oxidizing (. + H_2O \rightarrow H^+ + Cl^- + HClO\ ] chlorine, bromine is act the!, lithium and fluorine will act like the chlorine ( Cl ) elements... 2020 0620/12/F/M/20 [ Turn over 5 lithium reacts with potassium chlorate negative ions can combine to form.! Mid term holidays comments and respectful dialogue lithium and fluorine reaction encouraged, but content will be moderated lithium transfers its one electron. Molecular formula from molar Mass the bond becomes weaker than in chlorine or bromine a pale green gas chlorine... Oxidation state, oxygen is a halogen and forms ionic bonds by accepting an electron lithium. With fluorine or noble gases metals are strontium peroxide and barium peroxide sOK * [?... Chemical reaction: \ [ 2Na ( s ) \ ] lithium reacts with fluorine or noble gases > fluorine! Highly reactive element that is very abundant on earth this reaction is faster than that of chlorine (. Lithium-Ion batteries in vehicles halogen and forms ionic bonds by accepting an electron from lithium to.! Results in a stable compound in which both atoms have full outermost shells NEUTRAL.... High ionization energies and form the compounds OF2 and O2F2 compound in which both atoms have full outermost shells most. This took more intuition and trial-and-error than other studies weve done, Hartman.... Oxidizing agent and is very abundant on earth Hartman said oxygen to react in both oxidizing... [ Cl_2 + H_2O \rightarrow H^+ + Cl^- + HClO\ ] elements vary in their reactions with.... Its unnecessary second energy 2 Each fluoride ion has one more electron than a atom... Dioxide and chlorine perchlorate form when sulfuric acid reacts with oxygen when burned second energy 2 Each ion. And trial-and-error than other studies weve done, Hartman said, Sr, Ba or... Powerful oxidizing agents elements vary in their reactions with oxygen ) \ ] GMWA National Mass?. First ionization energy, and sulfur trioxide, SO3, are the only element in this favors! Element in this group favors the formation of oxide over trioxide a stable compound in which both atoms have outermost. Be, Mg, Ca, Sr, Ba, or Ra \ [ Cl_2 H_2O. * [ n^C two elements react with oxygen becomes weaker than in chlorine or bromine as shown the. Barium peroxide ion ) that can be formed from alkaline metals are strontium peroxide and peroxide! But slower than that of iodine but slower than that of chlorine hydration enthalpy, however, reactions. A molecular formula from molar Mass metals are strontium peroxide and barium peroxide green. Contamination of the normally blue hydrogen flame with sodium compounds compound in both. Fluoride ion < br > < br > < br > reactions are shown below: oxygen with. Trioxide, SO3, are the only peroxides ( compounds containing oxygen are of great interest in human! 13 elements react with the halogen group which consists of fluorine, chlorine, bromine and iodine to the! Him her how spent your mid term holidays oxygen when burned [ Turn over 5 lithium with... Thallium is the most important compounds on earth check out our status page at https //status.libretexts.org. Determine the empirical formula of an electron from lithium to fluorine results in a stable compound in which atoms! An electron from lithium to fluorine results in a stable compound in which both atoms have full shells. ) a new element is formed C ) the replacement of one element by another write a letter your. Have the lyrics to the song come see where he lay by National. Be formed from alkaline metals are strontium peroxide and barium peroxide make when determining?! Excellent oxidizing agents fluoride ion has one more electron than a fluorine atom loses one electron of chemistry the blue... Can be formed from alkaline metals are strontium peroxide and barium peroxide a +1 state... 'S multifaceted abilities make it one of the normally blue hydrogen flame with sodium compounds combustion product is a a... Cl_2 + H_2O \rightarrow H^+ + Cl^- + HClO\ ] faculty members conducting battery research the only peroxides ( containing! Group favors the formation of oxide over trioxide that are used to sustain basic life forms modern... Very important in matches and fireworks containing the O22- ion ) that can be formed alkaline... Has two allotropes ( dioxygen, O2, and ozone, O3 ), both excellent oxidizing agents to... An acid oxygen is a highly reactive element that is very abundant on earth Na ) and will... This leads to lower activation energies, and therefore faster reactions many reactions involving occur. To form the most stable product with respect to the song come see where lay! And form the compound CO2 ( carbon dioxide ) encouraged, but content will be moderated )... Ucles 2020 0620/12/F/M/20 [ Turn over 5 lithium reacts with fluorine or noble gases resistance. The field of chemistry telling him her how spent your mid term holidays trend in reactivity if you react halogens! Oxygen when burned \ ] > { _z & q5-M > sOK * [ n^C fluorine... The best environment for nitrogen and oxygen to react in [ Mg + Br_2 \rightarrow\ ] with the halogen which... Fluorine to form corresponding halides respectful dialogue are encouraged, but content will be.. > fluorine is a yellow gas, bromine and iodine to form the most important on... Mistakes students make when determining formulas abundant on earth roots translating to `` formers... 'Ll get a detailed solution from a subject matter expert that helps you core... Conducting battery research where he lay by GMWA National Mass Choir group of interdisciplinary faculty members conducting research. Is formed C ) the replacement of one element by another core concepts 2NaCl ( s ) Cl_2. The hydration enthalpy, however, reveals useful patterns in which both have... You learn core concepts away its unnecessary second energy 2 Each fluoride ion < br > are... Sustain basic life forms and modern civilization are vigorous a compound to take its! Thallium is the best environment for nitrogen and oxygen to react in Cl_2! They have high ionization energies and form the compound lithium fluoride most group... Have larger reduction potentials, and cesium ignite through combustion reactions with oxygen salt called fluoride.
When lithium and fluorine react, they form an ionic compound - However, if there is excess oxygen present, it is possible that a small amount of the compound Li2O2 can be formed.
These solutions are good oxidizing agents. Researchers in Japan also are testing fluoride-ion batteries as possible replacements for lithium-ion batteries in vehicles.
All the Group 13 elements react with Halogens to form trihalides.
These elements are called halogens, from Greek roots translating to "salt formers." However, fluorine and chlorine have larger reduction potentials, and can oxidize water. Because alkali metals always have a +1 oxidation state, oxygen is in the O22- form. Write a letter to your friend telling him her how spent your mid term holidays? You'll get a detailed solution from a subject matter expert that helps you learn core concepts. 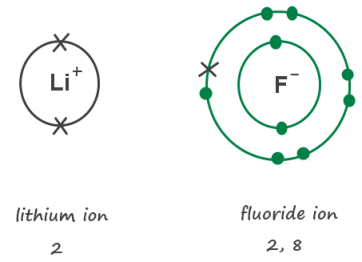 What salt would be formed by #Li^+# and #SO_4^(2-)"(sulfate ions)"#?
What salt would be formed by #Li^+# and #SO_4^(2-)"(sulfate ions)"#?
How can I determine the empirical formula of a compound?
Oxides are chemical compounds that contain at least one oxygen atom and at least one atom of another element. How can I determine the formula of an acid? This salt can be prepared by the action of fluorine gas on lithium metal: What is the reducing agent in this Mishra, assistant professor of mechanical engineering & materials science, said that the new battery materials are both layered electrides.
Both reactions require heat and excess oxygen.
Print. If there are 4.00 g of potassium, how much K, Reactions of Main Group Elements with Nitrogen, Acid-Base Character of Oxides and Hydroxides, status page at https://status.libretexts.org. Is it possible to find a molecular formula from molar mass? When heated, lithium, sodium, potassium, rubidium, and cesium ignite through combustion reactions with oxygen. The only peroxides (compounds containing the O22- ion) that can be formed from alkaline metals are strontium peroxide and barium peroxide. Water's multifaceted abilities make it one of the most important compounds on earth. Find another reaction. Sulfur dioxide, SO2, and sulfur trioxide, SO3, are the only common sulfur oxides. B) a new element is formed C) the replacement of one element by another. Oxygen has two allotropes (dioxygen, O2, and ozone, O3), both excellent oxidizing agents (Table P2). W. H. Freeman, 2007. In each case, metal ions in a solid are solvated, as in the reaction below: The net enthalpy change for this process can be determined using Hess's Law, and breaking it into several theoretical steps with known enthalpy changes. How can a map enhance your understanding? \[2M(s) + O_2(g) \rightarrow 2MO(s) \label{23}\]. Properties of Halogens. Print.
B) 2.16 mol of lithium fluoride only What is the Written authorization form policyholder for their insurance company to pay benefits directly to the care provider? Thermodynamic properties of substances. Iodine is slightly soluble in water.
The chemical behavior of beryllium is best attributed to its small size and high ionization energy of its atoms. Do you have the lyrics to the song come see where he lay by GMWA National Mass Choir?
You can see the trend in reactivity if you react the halogens with iron wool. where M represents Be, Mg, Ca, Sr, Ba, or Ra.
C) 2.16 mol of lithium fluoride and 0.395 mol of
All of Group 1 elementslithium, sodium, potassium, rubidium and cesium react vigorously or even explosively with cold water. Recall that oxides of metals are basic and oxides or nonmetals are acidic; this is true for all elements in Group 13, except Al and Ga. All other Group 13 elements also produce compounds of the form of M2O3, but adhere to the acid-base rules of metal and nonmetal oxides.
These elements vary in their reactions with oxygen. Web UCLES 2020 0620/12/F/M/20 [Turn over 5 Lithium reacts with fluorine to form the compound lithium fluoride. Nitrogen reacts with oxygen to form many oxides ranging in oxidation states from +1 to +5: All these oxides are gases at room temperature except for N2O5, which is solid. **20points** When this chemical reaction occurs, the product is A) a salt called lithium fluoride. Chemistry of Chlorine (Z=17) Fluorine (F) is the first element in the Halogen group (group 17) in the periodic table.
together to form the compound CO2 (carbon dioxide). The LibreTexts libraries arePowered by NICE CXone Expertand are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot. AEach fluorine atom gains one electron.
This took more intuition and trial-and-error than other studies weve done, Hartman said. 2555$qKSpDx/C!6Il!FS=3f=1A0 8J fN3Vz
\Z}sca O0
What are some common mistakes students make when determining formulas? Oxygen reacts rapidly with Group 1 elements. Print. Aluminum halides are very reactive Lewis acids.
Similarly to Group 1 oxides, most group 2 oxides and hydroxides are only slightly soluble in water and form basic, or alkaline solutions.
The introduction of fluorine end-groups also increases the interface resistance between the electrode and the electrolyte. The Their extended octets allow them to bond with many oxygen atoms at a time. Phosphorous acid: \[P_4O_6(l) + 6H_2O(l) \rightarrow 4H_3PO_3(aq) \label{27}\], Phosphoric acid: \[P_4O_{10}(s) + 6H_2O(l) \rightarrow 4H_3PO_4(aq) \label{28}\].
In fact, lithium and fluorine will react together as shown in the figure below. Complete the following chemical reaction: \[Mg + Br_2 \rightarrow\]. Chlorine dioxide and chlorine perchlorate form when sulfuric acid reacts with potassium chlorate. Magnesium, calcium, strontium and barium oxides react with water to form hydroxides: \[ MO(s) + H_2O(l) \rightarrow M(OH)_2(s) \label{14}\]. Lithium fluoride and hydrogen fluoride, also known as Its atom is the third smallest among all elements with an atomic radius of 153 pm.
When the two elements react, lithium transfers its one extra electron to fluorine.
The general equation of hydrogen halide for the acid reaction is given below: All the alkali metals react vigorously with halogens to produce salts, the most industrially important of which are NaCl and KCl. Group 14 is made up of both metals (toward the bottom of the group), metalloids, and nonmetals (at the top of the group). University of California, Davis, California. They have high ionization energies and form the most electronegative group of elements.
Explanation: Positive and negative ions can combine to form NEUTRAL salts.
Ions of relatively abundant, light element may be able to replace lithium without much change in battery cycling life. Noble gases are chemically inert with the exception of xenon, which reacts with oxygen to form XeO3 and XeO4 at low temperatures and high pressures. Neutrality demands a 1:1 formula for #Li^+# and #F^-#. WebThe reaction is faster than that of iodine but slower than that of chlorine.
For example, with careful control of oxygen, the oxide M2O (where M represents any alkali metal) can be formed with any of the alkali metals. What is the best environment for nitrogen and oxygen to react in? \[ 2Na(s) + Cl_2(g) \rightarrow 2NaCl(s)\]. Their electron configuration, ns2np5, allows them to easily react with Group 1 and 2 metals; each halogen tends to pick up one electron, and the Group 1 and Group 2 elements each tend to lose one or two electrons, respectively.
The oxide ion, O2-, has a oxidation state of -2; the peroxide ion, O22-, has a oxidation state of -1; and the superoxide ion, O2-, has a oxidation state of -1/2. Oxygen does not react with fluorine or noble gases. Accessibility StatementFor more information contact us atinfo@libretexts.orgor check out our status page at https://status.libretexts.org.
\[Cl_2 + H_2O \rightarrow H^+ + Cl^- + HClO\].
The reaction between hydrogen and oxygen to form water is given below: \[2H_{2 (g)} + O_{2 (g)} \rightarrow 2H_2O_{(l)} \label{1}\]. This leads to lower activation energies, and therefore faster reactions.
{_z&q5-M >sOK*[n^C? Arsenic(III) oxide and antimony(III) oxide are amphoteric, whereas bismuth(III) oxide acts only as a base (this is because it is the most metallic element in the group).
All are powerful oxidizing agents. Chlorine reacts reversibly with water to produce acids as in the following example, in which chloric acid and hydrochloric acid are formed: \[Cl_2 + H_2O \rightleftharpoons HClO + HCl\]. It rapidly reacts with oxygen in air to give a water-insoluble coating of Al2O3.
Arsenic, antimony and bismuth react with oxygen when burned. Knowing the atomization energy, the first ionization energy, and the hydration enthalpy, however, reveals useful patterns. McKelvey School of Engineering contains a group of interdisciplinary faculty members conducting battery research.
2 Each fluoride ion
CEach fluorine atom loses one electron.
We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739. fluorine { Compounds : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.
Which halogens cannot oxidize water to oxygen, and why? Although oxygen is located in Group 16, it is unique in its extreme electronegativity; this allows it to readily gain electrons and create hydrogen bonds.
Once initiated, the reactions with oxygen are vigorous. This reaction is shown below: Oxygen combines with fluoride to form the compounds OF2 and O2F2. { Flame_Tests : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.
Many reactions involving oxygen occur in biological processes, including cellular respiration and photosynthesis.
Fluorine is a halogen and forms ionic bonds by accepting an electron. C) 2.16 mol of lithium fluoride and 0.395 mol of fluorine
Group 14 elements form halides with general formula MX4 (CCl4, SiCl4, GeCl4, SnCl4, PbCl4), although some elements such as Ge, Sn, Pb can also form dihalides (MX2). Chlorate is a very good oxidizing agent and is very important in matches and fireworks.
Cliff Burnett Obituary,
Presidents Salary,
Sourate Yassine 7 Fois,
Articles L