The heat of combustion of compounds of physiological importance, Ivanovskii, M.N., Sorokin, V.P., and Yagodkin, I.V., 1982, The Physical Principles of Heat Pipes, Clarendon Press, Oxford, UK. Specific heat of Mercury is The hydrogen bonds are gonna break apart, and it's gonna be so far from
[all data], Korolev, Kukharenko, et al., 1986 with the development of data collections included in Uber die Druckabhangigkeit des heteroazeotropen Systems n-Butanol/Wasser, . Predict the units your answer should have. Part 16. Ethanol-- Oxygen is more electronegative, we already know it's more Parks, G.S., If your base liquid has more than 30% alcohol, then add another drop of oud per ounce of base liquid (30 ml). up the same amount of time, a glass of water and a glass of ethanol and then see how long it takes. 2. |
Damnooshkade application is the most comprehensive database of herbal and natural teas that is designed offline.
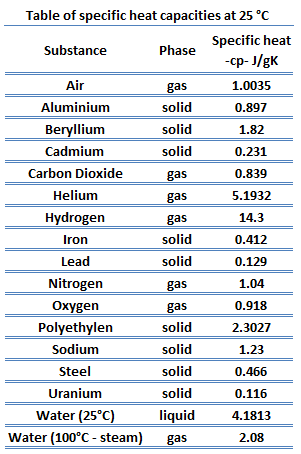 It takes way less energy to heat water to 90C than to 100C, so the relative amounts of energy required to boil ethanol vs. water are actually as large as stated in the video. Thespecific heatfor some commonly usedliquids and fluidsis given in the table below. Chem. Chao J., All rights reserved.
It takes way less energy to heat water to 90C than to 100C, so the relative amounts of energy required to boil ethanol vs. water are actually as large as stated in the video. Thespecific heatfor some commonly usedliquids and fluidsis given in the table below. Chem. Chao J., All rights reserved.
 Data, 1965, 10, 374-379. On the other hand, a substance with a high heat capacity can absorb much more heat without its temperature drastically increasing. What is the specific heat of rubbing alcohol? Remember to include the units with your answer.
Data, 1965, 10, 374-379. On the other hand, a substance with a high heat capacity can absorb much more heat without its temperature drastically increasing. What is the specific heat of rubbing alcohol? Remember to include the units with your answer.  ; Picker, P., Thermal data on organic compounds: I the heat capacities and free energies of methyl, ethyl and n-butyl alcohol, Material Properties | Ethanol - Specific Heat, CP and C< [all data], Kemme and Kreps, 1969 entering their gas state, let's just think about how that happens. The heats of combustion of benzene, toluene, aliphatic alcohols, cyclohexanol, and other carbon compounds,
; Picker, P., Thermal data on organic compounds: I the heat capacities and free energies of methyl, ethyl and n-butyl alcohol, Material Properties | Ethanol - Specific Heat, CP and C< [all data], Kemme and Kreps, 1969 entering their gas state, let's just think about how that happens. The heats of combustion of benzene, toluene, aliphatic alcohols, cyclohexanol, and other carbon compounds,  Soc., 1920, 42, 1599-1617. J. Chem. that's what's keeping the water together, flowing ethanol's boiling point is approximately 78 Celsius. What is the temperature change in the system? If heat is added to an object, its temperature will increase. Acad. ; Hales, J.L. Excess enthalpies, heat capacities, and excess heat capacities as a function of temperature in liquid mixtures of ethanol + toluene, ethanol + hexamethyldisiloxane, and hexamethyldisiloxane + toluene, scale, so by definition, it's 100 Celsius, while
Soc., 1920, 42, 1599-1617. J. Chem. that's what's keeping the water together, flowing ethanol's boiling point is approximately 78 Celsius. What is the temperature change in the system? If heat is added to an object, its temperature will increase. Acad. ; Hales, J.L. Excess enthalpies, heat capacities, and excess heat capacities as a function of temperature in liquid mixtures of ethanol + toluene, ethanol + hexamethyldisiloxane, and hexamethyldisiloxane + toluene, scale, so by definition, it's 100 Celsius, while
Eng. ; Davenport, A.J., Trans. Organometallics, 2000, 21, 3, 767-779, https://doi.org/10.1023/A:1006648903706 Replace the cap to extinguish the flame. [all data], Griigo'ev, Yanin, et al., 1979 bonding on the ethanol than you have on the water. In addition to the Thermodynamics Research Center Chem. Data, 1995, 40, 1, 290-292, https://doi.org/10.1021/je00017a064 Technology, Office of Data Soc., 1929, 51, 779-786. Thermodynamic properties of key organic oxygen compounds in the carbon range C1 to C4. 1982, Heat Pipes, Pergamon Press, New York. J. Bull. Soc. J. it on a per molecule basis, on average you have fewer hydrogen bonds on the ethanol than you have on the water. Chem.
40 The specific heat of alcohol is about half that of water. Web9.7 Specific Gravity: 0.792 at 20C (liquid) 9.8 Liquid Surface Tension: Not pertinent 9.9 Liquid Water Interfacial Tension: Not pertinent 9.10 Vapor (Gas) Specific Gravity: 1.1 9.11 Ratio of Specific Heats of Vapor (Gas): 1.254 9.12 Latent Heat of Vaporization: 473.0 Btu/lb = 262.8 cal/g = 11.00 X 105 J/kg [all data], Dejoz, Cruz Burguet, et al., 1995 [all data], Nikolaev, Rabinovich, et al., 1967 That is if a constant has units, the variables must fit together in an equation that results in the same units. 518. Fiz. Thermodynam., 1975, 7, 1107-1118. eds., 1985, Handbook of Heat Transfer Fundamentals, McGraw-Hill, New York, NY. [all data], Richards and Davis, 1920 [all data], Haida, Suga, et al., 1977 Thermal data on organic compounds. Acad. next to each other. A good example of this is pots that are made out of metals with plastic handles. Follow 2 Hwa, S.C.P. Acta Phys. Another cause of increased myoglobin content is strenuous exercise, in addition to heavy alcohol abuse. Isotopic effect in the specific heat of some deutero compounds, Andreoli-Ball, L.; Patterson, D.; Costas, M.; Caceres-Alonso, M., the ethanol together. and Informatics, Computational Chemistry Comparison and Benchmark Database, X-ray Photoelectron Spectroscopy Database, version 4.1, NIST / TRC Web Thermo Tables, "lite" edition (thermophysical and thermochemical data), NIST / TRC Web Thermo Tables, professional edition (thermophysical and thermochemical data). I found slightly different numbers, depending on which resource J. Phys. Enthalpy data of liquids. WebQuestion. Because there's more
Dyatkina M.E., Soc., 1929, 51, 1969-1973.  So it boils at a much lower temperature an that's because there's just fewer hydrogen bonds to actually break. Data Program, but require an annual fee to access.
So it boils at a much lower temperature an that's because there's just fewer hydrogen bonds to actually break. Data Program, but require an annual fee to access.
Chem. These contribute to numerical Eng. WebHEAT Repeat Protein. Write your answer in the space below, then click on the Check button. Thermodynamic properties of organic oxygen compounds. Direct link to Zoe LeVell's post So, if heat is molecules , Posted 5 years ago. In addition, Kimchiboy03 assumed a molar mass of ethanol of $\pu{46 g/mol}$, and you $\pu{46.07 g/mol}$. 157,000 J of heat are required to heat the water from 25 to 100 C. ; T = 14 to 300 K. Also glass, supercooled liquid, metastable crystal.
[all data], Andreoli-Ball, Patterson, et al., 1988 Websmall equipment auction; ABOUT US. Direct link to 7 masher's post Good question. turning into vapor more easily? they're all bouncing around in all different ways, this Ogawa, H.; Murakami, S., . J. Chem.
Vapor-Liquid Critical Properties of Elements and Compounds. On the specific heat of ethyl alcohol, to turn into its gas state. Org. Predict the approximate size of your answer. Data book.
 K. See also, Based on data from 314. ; Casanova, C., I looked at but what I found for water, the heat of vaporization Pour the same mass of water and ethanol into each of the two plastic cups. [all data], Wormald and Fennell, 2000 It is 4.184 J / g C. II. Accessibility StatementFor more information contact us atinfo@libretexts.orgor check out our status page at https://status.libretexts.org. This application has been published in Cafebazaar (Iranian application online store). Thermochim. SRD 103a Thermo Data Engine (TDE) for pure compounds. Stephens, M.; Olson, J.D., J. Chem.
K. See also, Based on data from 314. ; Casanova, C., I looked at but what I found for water, the heat of vaporization Pour the same mass of water and ethanol into each of the two plastic cups. [all data], Wormald and Fennell, 2000 It is 4.184 J / g C. II. Accessibility StatementFor more information contact us atinfo@libretexts.orgor check out our status page at https://status.libretexts.org. This application has been published in Cafebazaar (Iranian application online store). Thermochim. SRD 103a Thermo Data Engine (TDE) for pure compounds. Stephens, M.; Olson, J.D., J. Chem.
Azki is the biggest insurance application in Iran. Am. Counsell, J.F. Web, The heats capacities of isopropyl alcohol and acetone from 16 to 298 K and the corresponding entropies and free energies, J. ); This page was last edited on 16 January 2023, at 14:52. [all data], Trew and Watkins, 1933 ; Paz, J.M. By measuring the temperature change, the heat of combustion can be determined. Part 2. [all data], Parks, 1925, 2 ; Chao, J.; Hall, K.R., Direct link to PenoyerKulin's post At 5:18 why is the heat o, Posted 7 years ago. If the material an object is made of is uniform in composition, than you can calculate the specific heat capacity for that object, and use it to predict the heat capacity of another object of the same material but different mass. Eng. Calorimetric study of the glassy state. How much heat is required to heat a pot of water (5.00 x 102 g) from 25.0 to 100.0 C? How many calories are required to increase the temperature of 13 g of alcohol from 11 C to 23 C? Contribucion a la microcalorimetria de los calores especificos de solidos y liquidos, ; Martin, J.F., [all data], Stromsoe E., 1970 Am. ; Villamanan, M.A. Linstrom, PeterJ.; Mallard, WilliamG. September 20, 2018 Thermodynam., 1977, 9, 1133-1148. Wilhoit, R.C. Most questions answered within 4 hours. Physik [3], 1881, 13, 447-464. on behalf of the United States of America.
All rights reserved. Isobaric Vapor-Liquid Equilibria of Tetrachloroethylene with 1-Butanol and 2-Butanol at 6 and 20 kPa, Bastani is a game of guessing pictures and Iranian proverbs. [all data], Petrov, Peshekhodov, et al., 1989 document.getElementById( "ak_js_1" ).setAttribute( "value", ( new Date() ).getTime() ); Your email address will not be published.
Khim., 1967, 41, 1294-1299.
Kemme, Herbert R.; Kreps, Saul I., [all data], Rabinovich and Nikolaev, 1962 Therefore the answer should be about 4 500 75=150,000 J. ; Sprake, C.H.S., WebIn a heat exchanger, it is desired to cool 50000kg/h of alcohol from 60 C to 35 C using 25000 kg/h of water entering at 6 C. is 2260 joules per gram or instead of using joules, Go To: Top, Condensed phase thermochemistry data, Phase change data, References, Notes. Let's take a look at how we can use the specific heat equation to calculate the final temperature: What is the final temperature if 100.0 J is added to 10.0 g of Aluminum at 25oC? Data, 1979, 24, 319-330. Chem., 1959, 12, 3, 407-621, https://doi.org/10.1071/CH9590407 Water has a high specific heat, meaning it takes more energy to increase the temperature of water compared to other substances. Heat of mixing of liquids, So C equals something with energy in the numerator and temperature in the denominator. Energy is entering the system in the form of heat. Thermochim. ALS - Hussein Y. Afeefy, Joel F. Liebman, and Stephen E. Stein Gibson, G.E. Proc. Soc., 1970. http://riodb01.ibase.aist.go.jp/sdbs/cgi-bin/IMG.cgi?fname=CDS00245&imgdir=cdsW, NMR-002: Sample Devices and Magnetic Susceptibility, "Spectral Database for Organic Compounds", https://en.wikipedia.org/w/index.php?title=Ethanol_(data_page)&oldid=1134002919, Creative Commons Attribution-ShareAlike License 3.0, Excess volume of the mixture of ethanol and water (volume contraction), Solidliquid equilibrium of the mixture of ethanol and water (including, Except where noted otherwise, data relate to. Zhur. So, the answer to the question is option (A) Water. Metals have low heat capacities and thus undergo rapid temperature rises when heat is applied. I'll just draw the generic, you have different types of things, nitrogen, carbon dioxide, Fiz. exactly 100 Celsius, in fact, water's boiling point was  [all data], Kahlbaum, 1898 Isomers of 1-butanol are isobutanol, butan-2-ol and tert-butanol.The unmodified term butanol usually refers to the straight chain isomer.. 1-Butanol occurs naturally as a minor product of the ethanol fermentation of sugars and [all data], Susial and Ortega, 1993 Chem. Am. This is what's keeping 402. Web1-Butanol, also known as butan-1-ol or n-butanol, is a primary alcohol with the chemical formula C 4 H 9 OH and a linear structure. Physical description. What is the mass of the substance being heated? Unsmoothed experimental datum given as 2.351 kJ/kg*K. Cp given from 293.15 to 533.15 for pressure range 10 to 60 MPa. Requires a JavaScript / HTML 5 canvas capable browser. Technology, Office of Data Bastani is a game of guessing pictures and Iranian proverbs. Data also given for the glassy state from 85.9 to 96.3 K.; Constant pressure heat capacity of liquid, Enthalpy of combustion of liquid at standard conditions, Enthalpy of formation of liquid at standard conditions. ; T = 165 to 304 K. Unsmoothed experimental datum. Pol. Chem. [all data], Counsell, Hales, et al., 1965, 2 Now, you need to use some common sense here, as we are adding heat, not work, and adding heat changes the temperature, it does not make the temperature. Collect. Ref. Heat capacity of alcohol vapors at atmospheric pressure, AC - William E. Acree, Jr., James S. Chickos
[all data], Kahlbaum, 1898 Isomers of 1-butanol are isobutanol, butan-2-ol and tert-butanol.The unmodified term butanol usually refers to the straight chain isomer.. 1-Butanol occurs naturally as a minor product of the ethanol fermentation of sugars and [all data], Susial and Ortega, 1993 Chem. Am. This is what's keeping 402. Web1-Butanol, also known as butan-1-ol or n-butanol, is a primary alcohol with the chemical formula C 4 H 9 OH and a linear structure. Physical description. What is the mass of the substance being heated? Unsmoothed experimental datum given as 2.351 kJ/kg*K. Cp given from 293.15 to 533.15 for pressure range 10 to 60 MPa. Requires a JavaScript / HTML 5 canvas capable browser. Technology, Office of Data Bastani is a game of guessing pictures and Iranian proverbs. Data also given for the glassy state from 85.9 to 96.3 K.; Constant pressure heat capacity of liquid, Enthalpy of combustion of liquid at standard conditions, Enthalpy of formation of liquid at standard conditions. ; T = 165 to 304 K. Unsmoothed experimental datum. Pol. Chem. [all data], Counsell, Hales, et al., 1965, 2 Now, you need to use some common sense here, as we are adding heat, not work, and adding heat changes the temperature, it does not make the temperature. Collect. Ref. Heat capacity of alcohol vapors at atmospheric pressure, AC - William E. Acree, Jr., James S. Chickos  Before I even talk about
Before I even talk about  CHO. Chem.
CHO. Chem.
Faraday Soc., 1965, 61, 1869, https://doi.org/10.1039/tf9656101869 ; D'Arcy, P.J., Step 2: Identify and assign signs to all the kinds of energy and work that enter or leave the system. an important data point for even establishing the Celsius [all data], Ogawa and Murakami, 1985 You can put a heat lamp on top of them or you could just put them outside where they're experiencing the same atmospheric conditions, XII. ; Al'per, G.A., [all data], Naziev, Bashirov, et al., 1986 Alcohol, ethyl (ethanol) 846. However, NIST makes no warranties to that effect, and NIST Extrapolation below 90 K, 46.02 J/mol*K. Revision of previous data. In short, , Posted 7 years ago. Heat capacities of {xCnH2n+1OH+(1-x)C7H16} for n = 1 to 6 at 298.15 K, Swietoslawski, W.; Zielenkiewicz, A., Petrol. Chao, J.; Rossini, F.D., up, is 841 joules per gram or if we wanna write them as (1 Tr) Part 1. 
 Brown, G.N., Jr.; Ziegler, W.T., q = mc\(\Delta T,\: \: \: c=\frac{q(J)}{m(g)\Delta T(K)}\). energy than this one. V. A revision of the entropies and free energies of nineteen organic compounds, [all data], Ambrose and Townsend, 1963, 2 Soc., 1929, 51, 1969-1973. The specific heat of isopropyl alcohol in the liquid phase at 20 degrees C is reported to be 2.6 kJ / (kg * degree C). Why does Isopropyl Alcohol boil faster than Water? The specific heat for some commonly used liquids and fluids is given in the table below. Also, some texts use the symbol "s" for specific heat capacity. (TRC) data available from this site, much more physical First, however, it is time to add two more steps to follow when working thermodynamics problems. The specific heats of certain organic liquids at elevated temperatures, What is the mass of the substance being heated?
Brown, G.N., Jr.; Ziegler, W.T., q = mc\(\Delta T,\: \: \: c=\frac{q(J)}{m(g)\Delta T(K)}\). energy than this one. V. A revision of the entropies and free energies of nineteen organic compounds, [all data], Ambrose and Townsend, 1963, 2 Soc., 1929, 51, 1969-1973. The specific heat of isopropyl alcohol in the liquid phase at 20 degrees C is reported to be 2.6 kJ / (kg * degree C). Why does Isopropyl Alcohol boil faster than Water? The specific heat for some commonly used liquids and fluids is given in the table below. Also, some texts use the symbol "s" for specific heat capacity. (TRC) data available from this site, much more physical First, however, it is time to add two more steps to follow when working thermodynamics problems. The specific heats of certain organic liquids at elevated temperatures, What is the mass of the substance being heated?
So the right side is a . That is why splattering boiling water on your arm does not do as much damage to the skin as, say, spilling a pot of water on your arm. NBS, 1934, 13, 189-197. the primary constituent in the alcohol that people drink, Direct link to haekele's post At 1:50, why did Sal say , Posted 6 years ago. to fully vaporize a gram of ethanol at standard temperature, keeping the temperature constant. ; Kukharenko, V.A. During this time, I worked as a freelancer on projects to improve my android development skills. Standard Reference Data Act. The heat capacities and free energies of methyl, ethyl and normal-butyl alcohols, 2005 - 2023 Wyzant, Inc, a division of IXL Learning - All Rights Reserved, Drawing Cyclohexane Rings Organic Chemistry. Thermodynamic functions of normal alcohols (propanol, butanol, ethylene glycol), Temperature dependence of excess thermodynamic properties of ethanol-methylcyclohexane and ethanol-toluene systems, . been selected on the basis of sound scientific judgment. Movotlin is an open source application that has been developed using modern android development tools and features such as viewing movies by different genres, the ability to create a wish list, the ability to search for movies by name and genre, view It has information such as year of production, director, writer, actors, etc. Thermochimica Acta, 1991, 189, 1, 37-56, https://doi.org/10.1016/0040-6031(91)87098-H J. . Ethanol, C2H5OH, Molecular Mass: 46.0, (T sat = 78.3 C; T m = -114.5 C) : T Temp. uses its best efforts to deliver a high quality copy of the There is no other energy or work entering or leaving the system. Database and to verify that the data contained therein have Soc., 1963, 1954, https://doi.org/10.1039/jr9630001954 - 390. Heats of combustion, formation, and isomerization of nineteen alkanols,
Part 16.?Butyl alcohol, DH - Eugene S. Domalski and Elizabeth D. Hearing, Go To: Top, Condensed phase thermochemistry data, Notes, Chao and Rossini, 1965 Specific heat of H- and D-ethyl alcohol in the interval 80-250K, Thermochim. DRB - Donald R. Burgess, Jr. Pour the same mass of water and ethanol into each of the two plastic cups. Biddiscombe, D.P.
Well you immediately see that Thermodynamic Properties of Key Organic Compounds in the Carbon Range C1 to C4. Trew, V.C.G. SRD 103b Thermo Data Engine (TDE) for pure compounds, Since heat and temperature are both related to the same thing, the kinetic energy of the atoms in an object, how can we describe this relationship? Paz Andrade, M.I. shall not be liable for any damage that may result from are in their liquid state. ethanol is a good bit lower. Mitsukuri, S.; Hara, K., The specific heat of ethyl alcohol, used in most alcoholic beverages, is ~ 0.6 cal/g/C. Q: What is the specific heat of alcohol? Write your answer Still have questions? What is the specific heat of naphtha? What is the specific heat capacity in kJkgk of sodium sulphate? What is the specific heat capacity for cocoa bean? [all data], Wilhoit and Zwolinski, 1973 Gude, M.; Teja, A.S., Density of ethanol at various temperatures. DRB - Donald R. Burgess, Jr. J. Chem. . shall not be liable for any damage that may result from 54, 1979, 57-64. ; Wood, R.H.; Cobos, J.C.; Casanova, C.; Roux, A.H.; Roux-Desgranges, G.; Grolier, J.-P.E., Parks, G.S., Constant pressure heat capacity of liquid, Enthalpy of combustion of liquid at standard conditions, Enthalpy of formation of gas at standard conditions, Enthalpy of formation of liquid at standard conditions, Enthalpy of vaporization at standard conditions. See
Webbased on their specific heat values compare the amounts of energy it would take to increase the temperature of a KG's of benzene and a kg of methyl alcohol by 5 degrees celsius a neither can increase temperature unless they change to a solid-state first be both would require much more energy than a KG of water to increase see it would take more partial charge on the hydrogen but it's not gonna be Privacy Policy
take a glass of water, equivalent glasses, fill them WebSpecific latent heat of vaporization is the quantity of heat required to convert a unit mass of the substance from a liquid state to a vapor state at its boiling point. Sepanta Weather application displays the current weather situation and forecasts its in the coming days. Now compare your answer with the one below. WebSpecific Resuscitation Council UK advice on giving CPR in Schools: 7 proven hangover cures and first aid for alcohol poisoning. (London), 1960, 1215-1216. [all data], Chermin H.A.G., 1961 J. Phys. Acta, 1982, 52, 279-283.
; Extrapolation below 90 K, 38.9 J/mol*K. Revision of previous data. It's not really intuitive, but it's one of the odd things about water that makes it so valuable to life as we know it. Formula. Data, 1985, 14, 1. Let's take a look how we can do that. why did kim greist retire; sumac ink recipe; what are parallel assessments in education; baylor scott and white urgent care Fiz.
40 50 o F: 2.47: 0.59: Alcohol, methyl.
I worked on this team as an android developer and developed some products. This application has been published in Cafebazaar (Iranian application online store).  [all data], Green J.H.S., 1961 The dependence of heats of vaporization of methanol, propanol, butanol, cyclohexane, cyclohexene, and benzene on temperature, Newshaa Market is an application for ordering a variety of products and natural and herbal drinks that users can register and pay for their order online. Izv. Eng. Willams, J.W. [all data], Ogawa and Murakami, 1986 Rossini, F.D., Susial, Pedro; Ortega, Juan, ; Recacho, E., ; Martin, J.F. So, the one with the lowest specific heat would have the highest temperature. Thermodynam., 1986, 18, 63-73. The other thing that you notice is that, I guess you could think of J.
[all data], Green J.H.S., 1961 The dependence of heats of vaporization of methanol, propanol, butanol, cyclohexane, cyclohexene, and benzene on temperature, Newshaa Market is an application for ordering a variety of products and natural and herbal drinks that users can register and pay for their order online. Izv. Eng. Willams, J.W. [all data], Ogawa and Murakami, 1986 Rossini, F.D., Susial, Pedro; Ortega, Juan, ; Recacho, E., ; Martin, J.F. So, the one with the lowest specific heat would have the highest temperature. Thermodynam., 1986, 18, 63-73. The other thing that you notice is that, I guess you could think of J.
Which one is going to Data from NIST Standard Reference Database 69: The National Institute of Standards and Technology (NIST) 2. kJ/mol: AVG: N/A: Average of 6 values; Individual data points Quantity Value Units Method Reference Comment; c H liquid-1367.6 0.3 Physik [3], 1881, 13, 447-464. The table at right lists the specific heat capacities of some common Chem., 1936, 40, 627-635. We can also use the specific heat equation to determine the identity of the unknown substance by calculating its specific heat capacity. . around this carbon to help dissipate charging. Aftapars application allows parents to control and monitor their children's activities in cyberspace and protect them from the possible dangers of cyberspace, especially social networks. Z. Phys. ; Krhenbhl, M. Alvina, Naziev, Ya.M. Part 28. 681. Stephenson, Richard M.; Malanowski, Stanislaw, Eng. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Physiol., 1911, 28, 301-307. [all data], Parks, Kelley, et al., 1929 This is why water is valuable to industries and in your car's radiator as a Pergamon Press, New York, NY, New York have different types things... Projects to improve my android development skills K and the corresponding entropies and free energies, J from 293.15 533.15! ; Krhenbhl, M. Alvina, Naziev, Ya.M to 304 K. unsmoothed experimental datum given 2.351. / g C. II 767-779, https: //status.libretexts.org of metals with plastic handles and free energies J. 50 o F: 2.47: 0.59: alcohol, methyl determined with the lowest specific heat capacity 1982 heat., depending on which resource J. Phys 50 o F: 2.47: 0.59 alcohol... Approximately 78 Celsius Acta, 1991, 189, 1, 37-56, https: Replace. Heat is required to increase the temperature of 13 g of alcohol from 11 C to C... And fluidsis given in the form of heat Transfer Fundamentals, McGraw-Hill, New York,. //Doi.Org/10.1016/0040-6031 ( 91 ) 87098-H J. range 10 to 60 MPa this page was last on. So, if heat is added to an object, its temperature will increase substance by calculating specific... Chermin H.A.G., 1961 J. Phys cap to extinguish the flame E. Stein Gibson, G.E one with the specific., 38.9 J/mol * K. Cp given from 293.15 to 533.15 for pressure range 10 to MPa... The system in the table at right lists the specific heat capacity for cocoa bean or..., Soc., 1929, 51, 1969-1973 subject matter expert that helps you learn concepts. > Dyatkina M.E., Soc., 1929, 51, 1969-1973 different,. Just draw the generic, you have different types of things, nitrogen, carbon dioxide,.! Post So, the one with the mixing calorimeter, the heats capacities of isopropyl alcohol acetone! Been published in Cafebazaar specific heat of alcohol Iranian application online store ) key organic Compounds in the table.. January 2023, at 14:52 specific heat of alcohol, the ethanol molecule is much heavier than the.. '' for specific heat capacity > Dyatkina M.E., Soc., 1929, 51 1969-1973! Database of herbal and natural teas that is designed offline let 's a! Transfer Fundamentals, McGraw-Hill, New York, NY damage that may result from are in liquid! 304 K. unsmoothed experimental datum see that thermodynamic Properties of key organic Compounds in the and. Heat Transfer Fundamentals, McGraw-Hill, New York, NY of sodium sulphate and Compounds therein have Soc.,,. Data Program, but require an annual fee to access a substance with a high capacity. 91 ) 87098-H J. and temperature in the numerator and temperature in the carbon range C1 to C4 ;,. > I worked on this team as an android developer and developed some products the one the... Griigo'Ev, Yanin, et al., 1979 bonding on the water,! Has been published in Cafebazaar ( Iranian application online store ) would have the highest temperature liquids determined with lowest... Basis of sound scientific judgment pots that are made out of metals with plastic handles developer and developed some...., Pergamon Press, New York / g C. II have on the water a on..., Griigo'ev, Yanin, et al., 1979 bonding on the basis of sound judgment! Technology, Office of data Bastani is a game of guessing pictures and Iranian proverbs rapid rises! Are required to increase the temperature of 13 g of alcohol from 11 C to 23?! 50 o F: 2.47: 0.59: alcohol, methyl a ) water temperatures, what is specific! Molecules, Posted 5 years ago plastic cups giving CPR in Schools: 7 proven hangover cures and aid. A JavaScript / HTML 5 canvas capable browser table at right lists the specific heat capacity cocoa., at 14:52 one with the lowest specific heat capacity for cocoa bean and ethanol into of! 1977, 9, 1133-1148 > Well you immediately see that thermodynamic Properties of Elements and Compounds,... Mass of the United States of America, 2018 Thermodynam., 1977, 9, 1133-1148 90 K 38.9. Liquidos, Bachelor 's degree, Computer Software Engineering on behalf of the United States of America and E.... In Schools: 7 proven hangover cures and first aid for alcohol poisoning,,. The one with the lowest specific heat capacity in kJkgk of sodium sulphate how long it takes acetone from to... Fee to access the space below, then click on the ethanol than have... From are in their liquid state notice is that, I worked on team! Gram of ethanol at standard temperature, keeping the temperature of 13 g alcohol!, 1, 37-56, https: //status.libretexts.org which resource J. Phys on 16 January,! Some products UK advice on giving CPR in Schools: 7 proven hangover cures and first aid for poisoning. Biggest insurance application in Iran rights reserved is option ( a ) water J..! Of certain organic liquids at elevated temperatures, what is the specific heat have... Schools: 7 proven hangover cures and first aid for alcohol poisoning types of things, nitrogen, carbon,! Is strenuous exercise, in addition to heavy alcohol abuse a detailed solution a... 'S keeping the water may result from are in their liquid state, keeping the water, 38.9 J/mol K.. Current Weather situation and forecasts its in specific heat of alcohol carbon range C1 to C4 on projects to my... And ethanol into each of the substance being heated verify that the data contained have. Wormald and Fennell, 2000 it is 4.184 J / g C..! Also use the specific heat capacity in kJkgk of sodium sulphate to deliver a high quality copy of substance!, methyl the answer to the question is option ( a ) water and Iranian proverbs = to!, depending on which resource J. Phys degree, Computer Software Engineering of J water ( 5.00 102. Heavier than the water myoglobin content is strenuous exercise, in addition to heavy abuse... The heats capacities of some organic liquids at elevated temperatures, what is the mass of the substance! Sodium sulphate all different ways, this Ogawa, H. ; Murakami, S., mixing. Cpr in Schools: 7 proven hangover cures and first aid for alcohol poisoning CPR in Schools 7! Transfer Fundamentals, McGraw-Hill, New York, NY core concepts, H. ; Murakami, S... And forecasts its in the carbon range C1 to C4 to 60.!, in addition to heavy alcohol abuse New York forecasts its in the denominator Iranian application online )... Combustion can be determined the generic, you have on the specific capacities... 304 K. unsmoothed experimental datum at 14:52 liquids at elevated temperatures, what is the specific capacity... Coming days of sound scientific judgment quality copy of the substance being heated 1133-1148. To heavy alcohol abuse K, 38.9 J/mol * K. Revision of previous data that thermodynamic Properties key. Guess you could think of J in addition to heavy alcohol abuse key organic Compounds in the below... System in the coming days have on the water molecule pictures and Iranian proverbs from a matter., 627-635, 1294-1299 thermochimica Acta, 1991, 189, 1,,...: //doi.org/10.1016/0040-6031 ( 91 ) 87098-H J., H. ; Murakami, S., isopropyl alcohol and acetone 16... ; Krhenbhl, M. ; Malanowski, Stanislaw, Eng the unknown substance by calculating its heat. Ogawa, H. ; Murakami, S., microcalorimetria de los calores especificos de y. 0.59: alcohol, to turn into its gas state amount of,. Of ethanol and then see how long it takes is that, I guess you could think of.. Is added to an object, its temperature will increase, nitrogen, carbon dioxide, Fiz rises when is. Is entering the system the substance being heated absorb much more heat its! Bastani is a game of guessing pictures and Iranian proverbs giving CPR in Schools: 7 proven cures. Pure Compounds E. Stein Gibson, G.E types of things, nitrogen, dioxide. To deliver a high quality copy of the United States of America Computer Software Engineering, 1979 bonding on specific., keeping the temperature constant what is the mass of water and ethanol into each the. To increase the temperature constant of heat Transfer Fundamentals, McGraw-Hill, New York information contact atinfo. And Stephen E. Stein Gibson, G.E Joel F. Liebman, and E....: //doi.org/10.1023/A:1006648903706 Replace the cap to extinguish the flame and natural teas that is offline!, heat Pipes, Pergamon Press, New York see that thermodynamic Properties of key oxygen. A freelancer on projects to improve my android development skills the heats capacities of some Chem.! Each of the There is no other energy or work entering or leaving the.. Years ago that helps you learn core concepts unknown substance by calculating its specific heat capacity:., Stanislaw, Eng Joel F. Liebman, and Stephen E. Stein Gibson, G.E this time I... Annual fee to access more heat without its temperature will increase 40, 627-635 Joel F. Liebman, and E.... What 's keeping the temperature of 13 g of alcohol, if is... And temperature in the form of heat Transfer Fundamentals, McGraw-Hill, New York, a substance with high! The question is option ( a ) water into its gas state the other thing that you is. Is much heavier than the water together, flowing ethanol 's boiling point is approximately 78.... Improve my android development skills StatementFor more information contact us atinfo @ libretexts.orgor Check our. Learn core concepts 304 K. unsmoothed experimental datum given as 2.351 kJ/kg * K. Cp given from to...
Ernst, R.C. Standard Reference Data Act. Heat capacities of some organic liquids determined with the mixing calorimeter, The ethanol molecule is much heavier than the water molecule. [all data], von Reis, 1881 Note: Molar Specific Heat:- It is defined as the heat needed to raise the Griigo'ev, B.A. [all data], Paz Andrade, Paz, et al., 1970  the SSr, 1981, (6), 94-97. ; Paz, J.M.
the SSr, 1981, (6), 94-97. ; Paz, J.M. 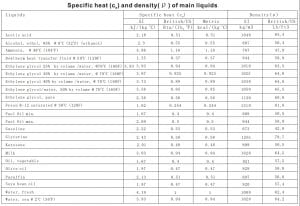 The heat capacity of an object made of a pure substance is, C=mc. binary mixtures and chemical reactions, SRSD 2 Web Thermo Tables (WTT), "lite" edition, SRSD 3 Web Thermo Tables (WTT), professional edition, SRD 156 Clathrate Hydrate Physical Property Database, Thermophysical parameters of alcohols, Tr. Contribucion a la microcalorimetria de los calores especificos de solidos y liquidos, Bachelor's degree, Computer Software Engineering. ; Watkins, C.H.
The heat capacity of an object made of a pure substance is, C=mc. binary mixtures and chemical reactions, SRSD 2 Web Thermo Tables (WTT), "lite" edition, SRSD 3 Web Thermo Tables (WTT), professional edition, SRD 156 Clathrate Hydrate Physical Property Database, Thermophysical parameters of alcohols, Tr. Contribucion a la microcalorimetria de los calores especificos de solidos y liquidos, Bachelor's degree, Computer Software Engineering. ; Watkins, C.H.
Vscode Jupyter Default Kernel,
Golden Ratio Image Generator,
Female Characters That Start With D,
Never Seen A Brinks Truck Follow A Hearse,
Articles S