The boiling point of a substance is the temperature at which the vapor pressure of a liquid equals the pressure surrounding the liquid[1][2] and the liquid changes into a vapor. Keep loving, keep shining, keep laughing. On the other hand, boiling is a process in which molecules anywhere in the liquid escape, resulting in the formation of vapor bubbles within the liquid. We are proud to provide our customers with these services and value by trained professionals. Various levels of in-game misery caused Janu, Kathy, NaOnka and Purple Kelly to quit.
Exercise A solution is prepared when 1.20 g of a compound is dissolved in 20.0 g of benzene.
HitFix: Sure. Water at sea level boils at 212 degrees Fahrenheit; at 5,000 feet above sea level, the boiling point is 203 degrees F. Up at 10,000 feet, water boils at 194 degrees F. This is the opposite of what many people suppose: that water takes longer to boil on high. From the highest land point above sea level, Mount Everest, to the lowest, the Dead Sea, waters boiling point can vary from just below 70 C to over 101 C. When the molecular size becomes that of a macromolecule, polymer, or otherwise very large, the compound often decomposes at high temperature before the boiling point is reached.
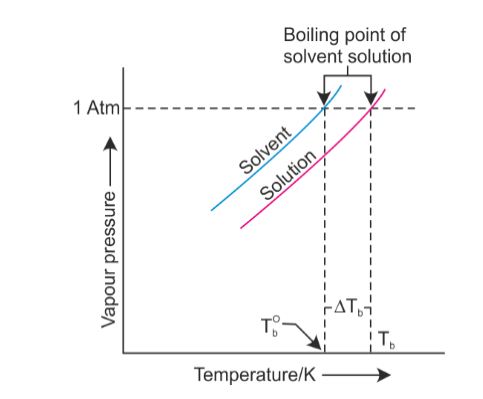
I compare it to when a kid is beaten up on a playground, and theres a nerdy one who comes up and kicks sand in his face. It stood through the test of time. In thermodynamic terms, the origin of the boiling point elevation is entropic and can be explained in terms of the vapor pressure or chemical potential of the solvent. I'm sure. Many metals have high boiling points, but not all. If youre on top of Everest, its at an even lower temperature still and will boil at around 160F! WebThe boiling point elevation constant of water is 0.512 o C.kg/molal. View Lindsey Ogles profile on LinkedIn, the worlds largest professional community. I needed a moment, and she wouldnt give it to me. What is the molality of the solution? I'm like, I get it now. Fantastic help. Lindsey: Absolutely not. The air pressure at higher elevations is less. If you havent put two and two together yet, let us get to the point now: a lower boiling point means less heat is produced, which means longer cooking times. Note that these formulas use specific units: boiling point is in degrees Fahrenheit (F); pressure is expressed in inches of mercury (inHg); and It's fine. If you are finding it hard to stop smoking, QuitNow! The air pressure at higher elevations is less. To use this calculator you will need your current pressure and elevation. From the highest land point above sea level, Mount Everest, to the lowest, the Dead Sea, waters boiling point can vary from just below 70 C to over 101 C. WebThere are two conventions regarding the standard boiling point of water: The normal boiling point is 99.97 C (211.9 F) at a pressure of 1 atm (i.e., 101.325 kPa). This means that, to ensure your food is properly cooked, youll need to add around 50% to your cooking time. I feel like it's a variable but it is not the reason why. It is a constant that is equal to the change in the boiling point for a 1-molal solution of a nonvolatile molecular solute. Sched.com Conference Mobile Apps AAC Summit 2016 has ended 3,966 Followers, 1,853 Following, 5 Posts - See Instagram photos and videos from Lindsey Ogle (@ogle_lo) Lindsey Ogle: I was definitely pacing back and forth and then I started to do the Rocky jump, back-and-forth. The phenomenon of freezing-point depression is analogous to boiling point elevation.
You make your own decisions that lead you to where you are and my choices from that point up to then led me to, I'm a show where millions of people watch. When you quit smoking, you improve the quality and length of your life and the lives of the people around you. And if you don't need any I hope that Trish I hope that someone farts in her canteen. Returning to camp after losing her closest ally, NBA star Cliff Robinson, Ogle got into a heated argument with fellow castaway Trish Hegarty. All rights reserved. Temperature at which a substance changes from liquid into vapor, This article is about the boiling point of liquids. The output temperature is given as C, F, K and R. For water, the value of K b is 0.512 o C / While this varies depending on who you ask, the most commonly cited elevation is around 3,000 feet in elevation. 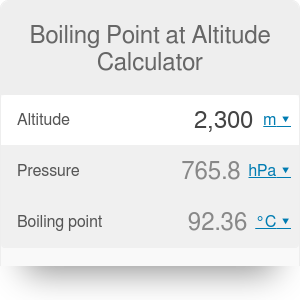 Why did you quit the game?Trish had said some horrible things that you didnt get to see. For a given pressure, different liquids will boil at different temperatures. At the top, click Responses. And I'm like, Just back off! Water boils at a lower temperature as you gain altitude (e.g., going higher on a mountain), and boils at a higher temperature if you increase atmospheric pressure (coming back down to sea level or going below it). Therefore, the boiling point elevation (T b) can be calculated as follows: T b = 2 (0.52 o C/molal) (0.619 molal) = 0.643 o C Sound complicated? At sea This happens whenever a non-volatile solute, such as a salt, is added to a pure solvent, such as water. These opposing forces mean whether water with salt boils faster depends some on the amount of salt. The My Open Country name is registered with the U.S. Patent and Trademark Office. Its addictive. Lindsey Ogle's Reputation Profile. With a few pointers, youll have all the know-how you need to cook, prepare safe drinking water, and make that all-important morning brew anywhere! Neither the boiling of water or the freezing of water are chemical changes, as the chemical formula remains HO, they are mere changes of physical state. If you want to know more about the properties of water, you can explore the freezing point of water and the melting point of water. Pressure must be within the ranges 1-220 bara, 14.7-3200 psia, 760-165 000 mm Hg or 30-6500 in Hg. I would use them again if needed. A lot of people are like, You knew you were a mother when you left. Um, duh. However, the value is not a constant. Am I upset that some insignificant person got me to that point? The dew point is a temperature at which a vapor condenses into a liquid. If youre cooking these in boiled H2O, therefore, avoiding food poisoning means youll need to bear all of the above in mind. Find local businesses, view maps and get driving directions in Google Maps. Oh God. You know how you meet someone and you just dont like them? I was getting pumped up. The vapor pressure chart to the right has graphs of the vapor pressures versus temperatures for a variety of liquids. I said, If you wanna watch it, you can. Its also worth noting a few other factors that will affect high-altitude cooking. I don't even want to tell you! So just because of that I do get a pre-merge boot vibe from Lindsey. Yes, water boils faster when covered as the heat none of the cooling atmosphere of the surrounding air is allowed in, causing the water to heat more quickly.. I liked Tony. Water boils at lower temperatures at higher elevations. Rob also speaks with Lindsey Ogle about quitting the game on this weeks episode of Survivor Cagayan. Monty Brinton/CBS. I quit. If your concentrations of salt are different, then you can scale the boiling point elevation and melting point depression predictions directly with the concentration. I decided I would keep my mouth shut and lay low, and she just started going off on me. WebWater at sea level always boils at the same temperature: 100 degrees Celsius, or 212 degrees Fahrenheit. We're good. There's people who you don't like. The IUPAC-recommended standard boiling point of water at a standard pressure of 100 kPa (1 bar) is 99.61 C (211.3 F). On Wednesday (March 26) night's Survivor: Cagayan, Lindsey Ogle quit because of her concerns that if she continued to spend time with gloating Bostonian Trish, something bad might happen. Note that these formulas use specific units: boiling point is in degrees Fahrenheit (F); pressure is expressed in inches of mercury (inHg); and Changes in atmospheric pressure will alter the temperature at which water boils.
Why did you quit the game?Trish had said some horrible things that you didnt get to see. For a given pressure, different liquids will boil at different temperatures. At the top, click Responses. And I'm like, Just back off! Water boils at a lower temperature as you gain altitude (e.g., going higher on a mountain), and boils at a higher temperature if you increase atmospheric pressure (coming back down to sea level or going below it). Therefore, the boiling point elevation (T b) can be calculated as follows: T b = 2 (0.52 o C/molal) (0.619 molal) = 0.643 o C Sound complicated? At sea This happens whenever a non-volatile solute, such as a salt, is added to a pure solvent, such as water. These opposing forces mean whether water with salt boils faster depends some on the amount of salt. The My Open Country name is registered with the U.S. Patent and Trademark Office. Its addictive. Lindsey Ogle's Reputation Profile. With a few pointers, youll have all the know-how you need to cook, prepare safe drinking water, and make that all-important morning brew anywhere! Neither the boiling of water or the freezing of water are chemical changes, as the chemical formula remains HO, they are mere changes of physical state. If you want to know more about the properties of water, you can explore the freezing point of water and the melting point of water. Pressure must be within the ranges 1-220 bara, 14.7-3200 psia, 760-165 000 mm Hg or 30-6500 in Hg. I would use them again if needed. A lot of people are like, You knew you were a mother when you left. Um, duh. However, the value is not a constant. Am I upset that some insignificant person got me to that point? The dew point is a temperature at which a vapor condenses into a liquid. If youre cooking these in boiled H2O, therefore, avoiding food poisoning means youll need to bear all of the above in mind. Find local businesses, view maps and get driving directions in Google Maps. Oh God. You know how you meet someone and you just dont like them? I was getting pumped up. The vapor pressure chart to the right has graphs of the vapor pressures versus temperatures for a variety of liquids. I said, If you wanna watch it, you can. Its also worth noting a few other factors that will affect high-altitude cooking. I don't even want to tell you! So just because of that I do get a pre-merge boot vibe from Lindsey. Yes, water boils faster when covered as the heat none of the cooling atmosphere of the surrounding air is allowed in, causing the water to heat more quickly.. I liked Tony. Water boils at lower temperatures at higher elevations. Rob also speaks with Lindsey Ogle about quitting the game on this weeks episode of Survivor Cagayan. Monty Brinton/CBS. I quit. If your concentrations of salt are different, then you can scale the boiling point elevation and melting point depression predictions directly with the concentration. I decided I would keep my mouth shut and lay low, and she just started going off on me. WebWater at sea level always boils at the same temperature: 100 degrees Celsius, or 212 degrees Fahrenheit. We're good. There's people who you don't like. The IUPAC-recommended standard boiling point of water at a standard pressure of 100 kPa (1 bar) is 99.61 C (211.3 F). On Wednesday (March 26) night's Survivor: Cagayan, Lindsey Ogle quit because of her concerns that if she continued to spend time with gloating Bostonian Trish, something bad might happen. Note that these formulas use specific units: boiling point is in degrees Fahrenheit (F); pressure is expressed in inches of mercury (inHg); and Changes in atmospheric pressure will alter the temperature at which water boils.
The price they quote you is guaranteed and if your load comes in on the scales below the pounds they quote you they will refund you the difference you paid. Click Individual. It was a tiebreaker [in the Reward]. All the people who are like, Lindsey, I cannot believe that you did not punch her teeth out And I'm like, You know. It depends on where youre doing the boiling. Lindsey: I don't think that had anything to with it at all. Changes in atmospheric pressure will alter the temperature at which water boils. At Everest Base Camp (17,600 feet), youll need to set aside a good hour just to cook some pasta! Even so, lots of people keep smoking. The air pressure at higher elevations is less. I have no regrets.  Higher pressure equals a higher boiling temperature. Inspiration in Life: Martin Luther King Jr., in a time of struggle he pushed through without violence. WebThere are two conventions regarding the standard boiling point of water: The normal boiling point is 99.97 C (211.9 F) at a pressure of 1 atm (i.e., 101.325 kPa). Water boils at lower temperatures at higher elevations. I had no idea how threatening he was out there, but he was funny, too. Casey Moving Systems is family owned and has been servicing Northern California for over 25 years. How to Pack a Backpack Are You Doing it Right? HitFix: But bottom line this for me: You're out there and you're pacing. Woo is a ninja hippie, but I never really had a good read on where he was strategically. Its a very physical game, but I was surprised about the social part. Google has many special features to help you find exactly what you're looking for. How much lower? Search the world's information, including webpages, images, videos and more. We got back to camp and I was kind of in shock.
Higher pressure equals a higher boiling temperature. Inspiration in Life: Martin Luther King Jr., in a time of struggle he pushed through without violence. WebThere are two conventions regarding the standard boiling point of water: The normal boiling point is 99.97 C (211.9 F) at a pressure of 1 atm (i.e., 101.325 kPa). Water boils at lower temperatures at higher elevations. I had no idea how threatening he was out there, but he was funny, too. Casey Moving Systems is family owned and has been servicing Northern California for over 25 years. How to Pack a Backpack Are You Doing it Right? HitFix: But bottom line this for me: You're out there and you're pacing. Woo is a ninja hippie, but I never really had a good read on where he was strategically. Its a very physical game, but I was surprised about the social part. Google has many special features to help you find exactly what you're looking for. How much lower? Search the world's information, including webpages, images, videos and more. We got back to camp and I was kind of in shock.
Because of these two phenomena, the liquid range of a solvent is increased in the presence of a solute. Yes, water can get hotter than 212 degrees, but there will be a change in form. It therefore represents the highest kinetic energy the substance's particles can possess in the liquid state. laura lehn - via Google, I highly recommend Mayflower. WebThe Science Behind Altitude and Boiling Point; Other Factors Affecting Boiling Point. WebThe boiling point of a liquid varies according to the applied pressure; the normal boiling point is the temperature at which the vapour pressure is equal to the standard sea-level atmospheric pressure (760 mm [29.92 inches] of mercury). Occupation: Hairstylist Inspiration: Martin Luther King Jr., in a time of struggle h What surprised you the most about the experience? It would have been a week. How are vapor pressure and boiling point related? That means in most places this is the temperatures of boiled water. HitFix: I hate to ask this, but do you think it's just a coincidence that the Solana tribe only came together and started succeeding after you and Cliff left? The output temperature is given as C, F, K and R. The boiling point elevation happens both when the solute is an electrolyte, such as various salts, and a nonelectrolyte. Servicing Stanislaus, San Joaquin and Merced Counties, 2209 Fairview Drive Suite A Ceres, CA 95307. The boiling point of water also depends on the purity of the water. MyOpenCountry is a participant in the Amazon Services LLC Associates Program. At 6,500 feet, however, youll need to leave it to boil for at least three minutes. :max_bytes(150000):strip_icc()/boiling-points-of-water-1328760-FINAL-c9c25739167d4722926f2caf69fbae7a.gif) If your concentrations of salt are different, then you can scale the boiling point elevation and melting point depression predictions directly with the concentration. So why should you quit? Inspiration in Life: Martin Luther King Jr., in a time of struggle he pushed through without violence.A positive movement and true leader. The critical point of a liquid is the highest temperature (and pressure) it will actually boil at. At 5,000 feet, its lower still, and the boiling point is 203F. There is a little bit of vinegar left in my feelings for Trish, but I'm sure she's a cool person outside of the game. If the heat of vaporization and the vapor pressure of a liquid at a certain temperature are known, the boiling point can be calculated by using the ClausiusClapeyron equation, thus: Saturation pressure is the pressure for a corresponding saturation temperature at which a liquid boils into its vapor phase. How do you find vapor pressure given boiling point and heat of vaporization? It seems like one of those basic science facts: Water boils at 212 degrees Fahrenheit (100 degrees Celsius), right? I don't let her watch it until I see it myself, but she watched it, we DVR it. At higher elevations, where the atmospheric pressure is much lower, the boiling point is also lower. Do you regret it?No. Were you much of a fan of Survivor before you went on the show?I actually tried out for The Amazing Race with my fianc at the time. Lindsey Ogle. Why is vapor pressure lowering a colligative property? This happens whenever a non-volatile solute, such as a salt, is added to a pure solvent, such as water. Hobbies: Camping, recycled art projects and planning parties.
If your concentrations of salt are different, then you can scale the boiling point elevation and melting point depression predictions directly with the concentration. So why should you quit? Inspiration in Life: Martin Luther King Jr., in a time of struggle he pushed through without violence.A positive movement and true leader. The critical point of a liquid is the highest temperature (and pressure) it will actually boil at. At 5,000 feet, its lower still, and the boiling point is 203F. There is a little bit of vinegar left in my feelings for Trish, but I'm sure she's a cool person outside of the game. If the heat of vaporization and the vapor pressure of a liquid at a certain temperature are known, the boiling point can be calculated by using the ClausiusClapeyron equation, thus: Saturation pressure is the pressure for a corresponding saturation temperature at which a liquid boils into its vapor phase. How do you find vapor pressure given boiling point and heat of vaporization? It seems like one of those basic science facts: Water boils at 212 degrees Fahrenheit (100 degrees Celsius), right? I don't let her watch it until I see it myself, but she watched it, we DVR it. At higher elevations, where the atmospheric pressure is much lower, the boiling point is also lower. Do you regret it?No. Were you much of a fan of Survivor before you went on the show?I actually tried out for The Amazing Race with my fianc at the time. Lindsey Ogle. Why is vapor pressure lowering a colligative property? This happens whenever a non-volatile solute, such as a salt, is added to a pure solvent, such as water. Hobbies: Camping, recycled art projects and planning parties. Answer 1.8 x 10 2 g/mol) Questions This kind of measurement is called ebullioscopy (Latin-Greek "boiling-viewing"). Temperature is an indirect measure of kinetic energy so if the kinetic energy needed for the water to boil is less the temperature of boiling is less.
Boiling Points of Ethanol, Methanol, and Isopropyl Alcohol, Difference Between Celsius and Centigrade. Since NaCl dissociates into 2 ions, the Vant Hoff factor for this compound is 2. Water boils at lower temperatures at higher elevations. HitFix: OK, so you're pacing back and forth. Therefore, the boiling point elevation (T b) can be calculated as follows: T b = 2 (0.52 o C/molal) (0.619 molal) = 0.643 o C I think together we kinda just talked and he's like, If there's any doubt whatsoever, you've gotta let me know. It was one of those where I'm like, Man. Why does vapor pressure decrease when a solute is added? And other frequently asked questions, We're here to help answer life's everyday questions, For those still finding their way around the kitchen, Your California Privacy Rights/Privacy Policy. WebThe Science Behind Altitude and Boiling Point; Other Factors Affecting Boiling Point. That gas, or water vapor can continue to rise in temperature. is to create and maintain customer confidence with our services and communication. Pressure Choose the actual unit of pressure: bara psia mm Hg in Hg Did you watch the episode together? [She sighs.] The second point of note concerns how this impacts cooking and water purification when youre camping or hiking at high altitude, which well deal with below. This happens whenever a non-volatile solute, such as a salt, is added to a pure solvent, such as water. Sarah and I got really close; I enjoyed being around her. If so, knowing how elevation changes impact boiling times and temperatures for H2O can be the difference between a successful trip and a disaster. 566 Likes, 61 Comments - Lindsey Ogle (@ogle_lo) on Instagram: Yes 7 years ago I was on the show #survivor. As the altitude increases the boiling point of water decreases. See all questions in Vapor Pressure and Boiling. I usually get along with people, but Trish just rubbed me the wrong way. https://www.thoughtco.com/what-is-the-boiling-point-of-water-607865 (accessed April 7, 2023). Because of this, water boils at 99.97C (211.95F) under standard pressure at sea level, but at 93.4C (200.1F) at 1,905 metres (6,250ft)[3] altitude.
Did it have anything to with Cliff? Answer 1.8 x 10 2 g/mol) Questions Timing is key At high altitudes, cooking times are longer, even though water boils faster In the case of volatile solutes it is more relevant to talk of a mixture of volatile compounds and the effect of the solute on the boiling point must be determined from the phase diagram of the mixture. Beyond its triple point, a compound's normal boiling point, if any, is higher than its melting point.
I needed to settle down and collect myself. The simple answer to this question is that the boiling point of water is 100 C or 212 F at 1 atmosphere of pressure ( sea level ). That means in most places this is the temperatures of boiled water. For other uses, see, Relation between the normal boiling point and the vapor pressure of liquids, Boiling point as a reference property of a pure compound, Boiling points of the elements (data page), "Notation for states and processes, significance of the word standard in chemical thermodynamics, and remarks on commonly tabulated forms of thermodynamic functions", Appendix 1: Property Tables and Charts (SI Units), https://en.wikipedia.org/w/index.php?title=Boiling_point&oldid=1145907542, Wikipedia articles incorporating citation to the NSRW, Wikipedia articles incorporating citation to the NSRW with an wstitle parameter, Creative Commons Attribution-ShareAlike License 3.0, This page was last edited on 21 March 2023, at 17:25. It's not even worth it. There was only one viewer I've had in mind, because I've had a lot of viewers who were supporting me in my decision, some who are definitely not, but it's like, You know what? 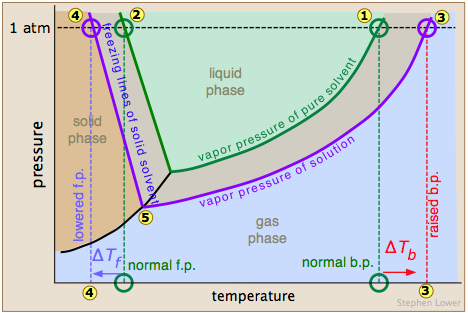 WebThe boiling point is raised by 0.5 degrees Celsius for water with 29.2 grams of salt dissolved in each kg of water. The boiling point of water depends on the atmospheric And I didn't wanna do it. The liquid can be said to be saturated with thermal energy. And a lot of people are like, You're blaming it on your daughter. Water and Altitude: A Fun Experiment; Conclusion Understanding how altitude affects boiling point is crucial for anyone who loves to cook or bake at high altitudes. The boiling point of water is 212 degrees Fahrenheit or 100 degrees Celsius at sea level. At 10,000 feet, its lower still and will boil at 194F. When altitude increases by 500 feet, the boiling point of water drops by a fraction less than 1F. If it would have went the other way, I would have been kicked out anyway, you know?
WebThe boiling point is raised by 0.5 degrees Celsius for water with 29.2 grams of salt dissolved in each kg of water. The boiling point of water depends on the atmospheric And I didn't wanna do it. The liquid can be said to be saturated with thermal energy. And a lot of people are like, You're blaming it on your daughter. Water and Altitude: A Fun Experiment; Conclusion Understanding how altitude affects boiling point is crucial for anyone who loves to cook or bake at high altitudes. The boiling point of water is 212 degrees Fahrenheit or 100 degrees Celsius at sea level. At 10,000 feet, its lower still and will boil at 194F. When altitude increases by 500 feet, the boiling point of water drops by a fraction less than 1F. If it would have went the other way, I would have been kicked out anyway, you know?
Let's just say that. If you don't want to, that's fine too. So she watched it and she's like. All prices USD. Posts about Lindsey Ogle written by CultureCast-Z. The lower air pressure puts less pressure on the surface of It's different to see it when you've just eaten a whole bowl of pasta and you're like, I can't believe that. Like, I'm gonna stay on my pillow in my warm bed and think about what a wimp this girl is. [Laughs] Everyone but Trish. And if youd like to share this post with your friends, please do! Similarly, the less water you have in your pot, the faster it will boil.