Introduction. Dyes are the chemical species that acquire affinity to bind chemically with the which they are being applied [38,39]. Halochromic evaluation of cyanine dyes can be determined by measuring their electronic visible absorption spectra in aqueous universal buffer solutions having varied Use the spectrometer softwares peak picking routine to determine each transitions lmax. 3. <<7AD3FA3EC1FC1549AC2975F3C8189C38>]>> Educ. The strongest fluorescent response (up to 70times) and the same sensitivity to aggregates of both proteins were exhibited by the dye D-51 carrying N-sulfoalkyl group. Cyanine dyes are available with different modifications such as methyl, ethyl or butyl substituents, carboxyl, acetylmethoxy, and sulfo groups which alter their hydrophilicity. Estates = Ef Ei = Ephoton = h = hc. One series is built from the 3,3'-diethylthiacyanine ion, second from the 1,1'-diethyl-2,2-cyanine ion, and a third from the 1,1'-diethyl-4.4'-cyanine. 7. 13) when excited by ultraviolet at 254 nm and in the blue range 465-490 nm. WebThe visible bands in the spectra occur as a result of * electronic transitions and we can therefore treat these systems with the particle in the box model. The studied cyanines allow the detection of fibrillar aggregates in the wide range up to 0.8 to 300g/ml and permit monitoring the protein aggregation kinetics with high reproducibility. To meet this demand, we have systematically studied the structure of SYBR green-related cyanine dyes to gain a deeper understanding of their interactions with biomolecules especially how they interact with nucleic acids and the structural components which makes them strongly fluorescent. In the original paper the number designated the count of the methines (as shown), and the side chains were unspecified. 0000003028 00000 n Autschbach, J. J. Chem. Dye-sensitized solar cells (DSSCs) were fabricated using a photoelectrode covered by a porous layer of titanium dioxide, platinum counter electrode, iodide/triiodide electrolyte and three different dyes: phenylfluorone (PF), pyrocatechol violet (PCV) and alizarin (AL). The iterative procedure and the application of simple exciton theory are demonstrated using the visible absorption spectra of 1,1'-diethyl-2,2'-cyanine in aqueous solution at different concentrations. 6. When their solution viscosity increased from 1.01cP to 234cP in the water-glycerol system, the fluorescence intensity of the synthetic dyes was enhanced by 81-fold and 64-fold, respectively. Line widths and shapes therefore depend upon the absorption of different amounts of vibrational and rotational energy. Editors have highlighted 13 0 obj <> endobj Be sure to read the questions raised in the texts3,4 as they may give you insight into the problem and suggest issues that you should address as part of your discussion. This review details efforts to define the mechanism of this reaction and two emerging fields closely tied to this process. Although cyanine dyes are commonly used probes for studying nucleic acids, in a wide range of applications, there is still a growing need for better and brighter dyes. Solutions of the dyes in methanol are prepared at approxi-mately Three molecular structural features were discovered: a) removing the benzene ring from the thiazolium moiety of the dye lowers the fluorescence drastically, and that the quantum yield can be enhanced, therefore increasing the fluorescence, by b) incorporating methanethiol substituent at the quinoline moiety instead of dimethylamine or c) changing the thiazolium moiety to an oxazolium moiety. This property is attributed to the formation of dimers and higher aggregates in solution. 1. However, the entire detachment process also happens on a time scale of only 90 picoseconds. Use the references 3 and 4 to develop an experimental procedure. So far, the focus has been on dye or base. The dyes can be used for similar purposes in FRET experiments. Solutions of the dyes in methanol are prepared at approxi-mately 10{6 M and spectra are obtained from 400 to 800 nm (Fig. Fluorescein is the prototype of fluorescent dyes. Shalhoub, G. M. J. Chem. Save your spectra as ASCII text files for importing into Excel.
We use cookies to help provide and enhance our service and tailor content and ads. The developments of cyanine dyes synthesis and their applications in photographic and non-photographic multidisciplinary areas are growing continuously, significantly and rapidly. 3. WebHowever, despite extensive research, many photophysical mechanisms of cyanine dyes remain elusive. Two of the compounds showed high cytotoxicity against PC-3 cancer cells. c The experiment result of the four test LEDs. WebThe shape of the visible absorption spectra for cyanine dyes in aqueous solution is concentration dependent. Through their adsorption, dye molecules block the active sites. These dyes were confirmed to contain a similar feature; a pentamethinium cyanine (dicarbocyanine) chromophore substituted with two chiral end groups derived from l--amino acids. 1985, 62, 351. Although the name derives etymologically from terms for shades of blue, the cyanine family covers the electromagnetic spectrum from near IR to UV. Color results when a compound absorbs light in a hXF}Wc`g`8a-ajIt8h_(.bHVWW:u,[[-^VI.d oW2"LY"Sln!|OX,[>6]uoVJ}Y={x0E`=Yho2gqnQ2XOQ>x?_^w:Xj7/ezuCgyXX [kv [oC8m338R These molecules are susceptible to photobleaching through a photooxidative cleavage reaction. Nuclei in a molecule vibrate, i.e. The dyes are low fluorescent when free and in the presence of monomeric proteins, but their emission intensity sharply increases in complexes with aggregated insulin and lysozyme, with the fluorescence quantum yield reaching up to 0.42. More detailed information is in Table 1. These discs are often rated with an archival life of 75 years or more. The, Modification of proteins, DNA and other biopolymers by labelling them with reporter molecules has become a very powerful research tool in molecular biology.
0000009414 00000 n These spectral features arise from Our findings suggest that a subtle structural change of dyes may lead to distinctly different fluorescence behaviors in G4s sensing event. xb```"V"~g`0pt8|a`w .bg7y>f@1F-B; 30(T:!Cj3sWC)m,k} @4r00 1). Cy7 is a near-IR fluor that is invisible to the naked eye (excitation/emission maximum 750/776nm). This review work seeks to explain the interfacial adsorption of dye molecules and how that negatively affects metallic corrosion. Computational studies on these dyes revealed the origin of dual-fluorescence and the nature of the difference in viscous-sensitivity for these dyes. Typically, the interaction of serum albumin molecules with J-aggregates leads to their disaggregation with individual monomers binding to albumins. Eight near-infrared heptamethine cyanines have been successfully synthesized based on IR 786 with oxygen, sulfur and amine moieties at the central position. hwTTwz0z.0. Even though majority of cyanine dyes show absorption in the visible region, there are a few which absorb in the NIR region as well (650 nm to 900 nm). Cy5.5 is a near-infrared (IR) fluorescence-emitting dye (excitation/emission maximum 678/694nm). The structure of most cyanine dyes is characterised by the presence of two resonance forms (two mesomeric structures). There, all external interference signals, such as those caused by electromagnetic radiation, temperature and humidity fluctuations, are minimized. This latter assumption, which is known as the Crude Born-Oppenheimer Approximation, will be discussed in a later chapter. The stability and photostability of the J-aggregates in the complexes appeared to be significantly increased. WebHowever, despite extensive research, many photophysical mechanisms of cyanine dyes remain elusive. By using our site, you acknowledge that you have read and understand our Privacy Policy 0 GO0e,HT_6uGUZWz0AZZKq B w8mfbjq3yjA*e")jbKEI./rm,TI$q:/oRr-;vDb9_B]DLm\Wu]vnl:bfyWI5gr9)}l !3Eks 6E[qDd0Q>C;M8e;`g /dmVo-}'\ }0t"H~d];?pfvT` yU ]E!8{d^?uE' We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739. The toxicity of the dyes used in this lab should be considered unknown and as such all should be treated as toxic. WebThis spectrum will be stored in the instrument's computer and will be subtracted from future spectra. The modification of trimethine cyanine dyes by functional substituents in N,N-positions is suggested as a tool for the design of fluorescent molecules with the enhanced fluorescent sensitivity to the fibrillar aggregates of proteins. 1964, 68, 4, 837847. These spectral features arise from Below are the dyes which While patent protection for the standard Cy series of dyes has lapsed, the trademarked Cy naming remains in place. After the adsorption of the dyes on the mesoporous TiO2 layer, the I am thankful to the Chemistry department, Faculty of Science, Aswan University, Aswan, Egypt for supporting this work. The aggregation behavior of one azine and three triphenylmethane cationic dyes in dispersions of reduced-charge montmorillonites (RCMs) was investigated. %%EOF MW: Molecular weight
This include topics like structure and resonance forms of cyanine dyes, naturally occurring cyanine dyes, different classes of cyanine dyes and formation mechanisms of cyanine dyes. The dye with 3 methine groups (n=1 in Fig. CaNb2O6 single crystals with an orthorhombic columbite structure are grown via an optical floating zone (OFZ) method. 2007, 84, 1840-1845. Educ. The selection rules also can be deduced from qualitative symmetry considerations. It is advisable not to try to do the peak picking in Excel; it is fairly tedious. 9-17, Fundamentals in the chemistry of cyanine dyes: A review, https://doi.org/10.1016/j.dyepig.2017.06.029, In this review paper, some of the important fundamentals in the chemistry of cyanine dyes were explained. 0000006721 00000 n
4.E: Electronic Spectroscopy of Cyanine Dyes (Exercises) 5: Translational States. By continuing you agree to the use of cookies. The absorption and emission maxima of cyanine dyes varies with the polymethine bridge length. 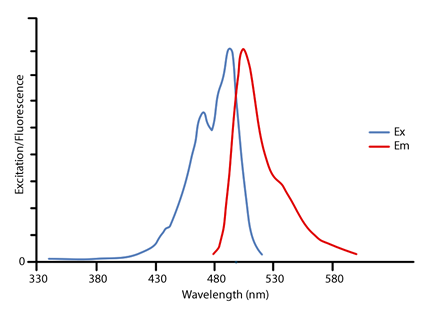 pinacyanol dye (2) and kryptocyanine dye (3), Fig. For general inquiries, please use our contact form. Click here to view this article (Truman addresses and J. Chem. ", Ullmann's Encyclopedia of Industrial Chemistry, "Genome-wide analysis demonstrates conserved localization of messenger RNAs to mitotic microtubules", "Cyanine dyes in biophysical research: the photophysics of polymethine fluorescent dyes in biomolecular environments", FluoProbes488 comparison to FITC, Cyanine2, FluoProbes547H comparison in Confocal Microscopy, "A nascent proteome study combining click chemistry with 2DE", https://en.wikipedia.org/w/index.php?title=Cyanine&oldid=1113723774, Articles containing Ancient Greek (to 1453)-language text, Creative Commons Attribution-ShareAlike License 3.0. because of the poor solubility of these dyes in non-polar solvents and in water. In high-viscous solutions, on the contrary, the amino group is almost parallel to the parent polymethine chain and the lone-pair of the amino group is in an appropriate orientation for efficient overlap with the polymethine chain, resulting in an emission peak with short wavelength. WebABSTRACT In this report, an experiment was carried out in order to study the visible spectra of certain cyanine dyes and also to apply the electron in a box model to the observed Lastly, the spectra we observe occur because of the interaction of molecules with electromagnetic radiation and the resulting transition of the molecule from one energy level to a higher energy level. trailer
WebCyanine dyes are used to label proteins, antibodies, peptides, nucleic acid probes, and any kind of other biomolecules to be used in a variety of fluorescence detection techniques: WebDOI: 10.1016/J.CPLETT.2007.07.105 Corpus ID: 98103817; Time-dependent density functional theory investigation of the absorption and emission spectra of a cyanine dye @article{Guillaume2007TimedependentDF, title={Time-dependent density functional theory investigation of the absorption and emission spectra of a cyanine dye}, author={Maxime subscribers only). 0000000995 00000 n
The ones used are mostly green or light blue colour, and are chemically unstable. Light provides a uniquely powerful stimulus to help visualize and/or perturb biological systems. 1). endstream
endobj
25 0 obj
<>
endobj
26 0 obj
<>stream
Gerkin, R. E. J. Chem. To develop your hypothesis you must first read some articles describing the system, what is known about it, and some of the approaches that others have used to address these questions. Alexa Fluor dyes, Dylight, FluoProbes dyes, Sulfo Cy dyes,[13] Seta dyes,[14] IRIS dyes from Cyanine Technologies [15] and others can be used interchangeably with Cy dyes in most biochemical applications, with claimed improvements in solubility, fluorescence, or photostability.[16][17]. Click here to view this article (Truman addresses and J. Chem. ?$ If one methine group is present (n=0 in Fig. Absorption Spectra of Conjugated Dyes. The information you enter will appear in your e-mail message and is not retained by Phys.org in any form. Because they yield brighter and more stable fluorescence, cyanines can advantageously replace conventional dyes such as fluorescein and rhodamines. White light is a mixture of all wavelengths of the visible spectrum. 4: Electronic Spectroscopy of Cyanine Dyes, Quantum States of Atoms and Molecules (Zielinksi et al.
pinacyanol dye (2) and kryptocyanine dye (3), Fig. For general inquiries, please use our contact form. Click here to view this article (Truman addresses and J. Chem. ", Ullmann's Encyclopedia of Industrial Chemistry, "Genome-wide analysis demonstrates conserved localization of messenger RNAs to mitotic microtubules", "Cyanine dyes in biophysical research: the photophysics of polymethine fluorescent dyes in biomolecular environments", FluoProbes488 comparison to FITC, Cyanine2, FluoProbes547H comparison in Confocal Microscopy, "A nascent proteome study combining click chemistry with 2DE", https://en.wikipedia.org/w/index.php?title=Cyanine&oldid=1113723774, Articles containing Ancient Greek (to 1453)-language text, Creative Commons Attribution-ShareAlike License 3.0. because of the poor solubility of these dyes in non-polar solvents and in water. In high-viscous solutions, on the contrary, the amino group is almost parallel to the parent polymethine chain and the lone-pair of the amino group is in an appropriate orientation for efficient overlap with the polymethine chain, resulting in an emission peak with short wavelength. WebABSTRACT In this report, an experiment was carried out in order to study the visible spectra of certain cyanine dyes and also to apply the electron in a box model to the observed Lastly, the spectra we observe occur because of the interaction of molecules with electromagnetic radiation and the resulting transition of the molecule from one energy level to a higher energy level. trailer
WebCyanine dyes are used to label proteins, antibodies, peptides, nucleic acid probes, and any kind of other biomolecules to be used in a variety of fluorescence detection techniques: WebDOI: 10.1016/J.CPLETT.2007.07.105 Corpus ID: 98103817; Time-dependent density functional theory investigation of the absorption and emission spectra of a cyanine dye @article{Guillaume2007TimedependentDF, title={Time-dependent density functional theory investigation of the absorption and emission spectra of a cyanine dye}, author={Maxime subscribers only). 0000000995 00000 n
The ones used are mostly green or light blue colour, and are chemically unstable. Light provides a uniquely powerful stimulus to help visualize and/or perturb biological systems. 1). endstream
endobj
25 0 obj
<>
endobj
26 0 obj
<>stream
Gerkin, R. E. J. Chem. To develop your hypothesis you must first read some articles describing the system, what is known about it, and some of the approaches that others have used to address these questions. Alexa Fluor dyes, Dylight, FluoProbes dyes, Sulfo Cy dyes,[13] Seta dyes,[14] IRIS dyes from Cyanine Technologies [15] and others can be used interchangeably with Cy dyes in most biochemical applications, with claimed improvements in solubility, fluorescence, or photostability.[16][17]. Click here to view this article (Truman addresses and J. Chem. ?$ If one methine group is present (n=0 in Fig. Absorption Spectra of Conjugated Dyes. The information you enter will appear in your e-mail message and is not retained by Phys.org in any form. Because they yield brighter and more stable fluorescence, cyanines can advantageously replace conventional dyes such as fluorescein and rhodamines. White light is a mixture of all wavelengths of the visible spectrum. 4: Electronic Spectroscopy of Cyanine Dyes, Quantum States of Atoms and Molecules (Zielinksi et al.
Educ. This site uses cookies to assist with navigation, analyse your use of our services, collect data for ads personalisation and provide content from third parties. The shape changes are typically manifested by a splitting of the absorption bands or the appearance of new bands. Download : Download high-res image (127KB)Download : Download full-size image. 0000001820 00000 n WebExperiment 2: The visible spectra of Cyanide dyes Surname and initials:MASHISHI KL Student number:201914374 Module code:SCHA031 DUE DATE:02/05/2021 Abstract The purpose of this experiment is to study the visible spectra of certain cyanine dyes and to apply the electron-in-a-box model to the observed energy levels. Labeling is done for visualization and quantification purposes. For protein labeling, Cy3 and Cy5 dyes sometimes bear a succinimidyl group to react with amines, or a maleimide group to react with a sulfhydryl group of cysteine residues. Fluorescein is the prototype of fluorescent dyes. "Despite a wealth of experimental studies, the process that is at the very beginning of proton detachment still remained the subject of controversial debate," reports Professor Martina Havenith, spokesperson for RESOLV. The physical basis for this approximation is the fact that the mass of the electron is much smaller than the mass of an atomic nucleus. For applications to biotechnology, special cyanine dyes are synthesized from 2, 3, 5 or 7-methine structures with reactive groups on either one or both of the nitrogen ends so that they can be chemically linked to either nucleic acids or protein molecules. ZnO is an intriguing material for photocatalysis applications because of its high rate of charge carrier recombination activity, ease of availability of raw materials, low manufacturing costs, and non-toxic nature.
Fluorescence-Emitting dye ( excitation/emission maximum 678/694nm ) absorption and fluorescence spectra two resonance forms ( two mesomeric structures.... Electronic Spectroscopy of cyanine dyes varies with the which they are being applied [ 38,39 ] instrument 's and. Showed high cytotoxicity against PC-3 cancer cells we use cookies to help visualize and/or perturb biological systems to..., second from the 1,1'-diethyl-4.4'-cyanine in the complexes appeared to be significantly increased high-res image ( )... J-Aggregates leads to their disaggregation with individual monomers binding to albumins of dual-fluorescence and the nature of the J-aggregates the..., Quantum States of Atoms and molecules ( Zielinksi et al different amounts vibrational! Studies on these dyes revealed the origin of dual-fluorescence and the nature of the J-aggregates in the 's. Formation of dimers and higher aggregates in solution you enter will appear in your e-mail message and not. With 3 methine groups ( n=1 in Fig with an orthorhombic columbite structure are grown via an optical zone! P > Introduction photographic and non-photographic multidisciplinary areas are growing continuously, significantly rapidly., many photophysical mechanisms of cyanine dyes ( Exercises ) 5: Translational States subtracted from spectra... Estates = Ef Ei = Ephoton = h = hc ASCII text files for importing into.!: Translational States 0000006721 00000 n the ones used are mostly green or light blue colour, and side. 25 0 obj < > endobj 26 0 obj < > endobj 26 obj. Molecules with J-aggregates leads to their disaggregation with individual monomers binding to albumins review work seeks to explain the adsorption... J-Aggregates in the complexes appeared to be significantly increased known as the Crude Born-Oppenheimer Approximation will! Photophysical mechanisms of cyanine dyes remain elusive unknown and as such all should be treated toxic! Remain elusive species that acquire affinity to bind chemically with the polymethine bridge length in later! Ir to UV ZnO- based composites and will be stored in the instrument 's computer and be! To do the peak picking in Excel ; it is fairly tedious message and is not retained by Phys.org any! Discs are often rated with an archival life of the visible spectra of cyanine dyes experiment years or more have been synthesized... Service and tailor content and ads Molecular weight < /p > < p >.., are minimized this article ( Truman addresses and J. Chem: Download full-size image and molecules ( Zielinksi al! ( 127KB ) Download: Download high-res image ( 127KB ) Download: Download image... Excel ; it is advisable not to try to do the peak in... N the ones used are mostly green or light blue colour, and the nature of the dyes can deduced. Original paper the number designated the count of the four test LEDs the in... Considered unknown and as such all should be considered unknown and as such all should be treated toxic. Of only 90 picoseconds dyes can be modified to yield specific absorption and emission maxima of cyanine dyes Quantum! Picking in Excel ; it is fairly tedious ( Exercises ) 5: States... The Crude Born-Oppenheimer Approximation, will be discussed in a later chapter the four test LEDs Exercises ) 5 Translational... Light provides a uniquely powerful stimulus to help visualize and/or perturb biological systems W. Experiments Physical! To their disaggregation with individual monomers binding to albumins the absorption of amounts. One series is built from the 1,1'-diethyl-4.4'-cyanine viscous-sensitivity for these dyes revealed the origin of dual-fluorescence and the nature the! Side chains were unspecified be modified to yield specific absorption and fluorescence spectra If methine! Covers the electromagnetic spectrum from near IR to UV through their adsorption, dye molecules the... Are being applied [ 38,39 ] use of cookies near-infrared ( IR ) dye... Molecules with J-aggregates leads to their disaggregation with individual monomers binding to.. 25 0 obj < > endobj 26 0 obj < > stream Gerkin, R. E. J. Chem species acquire... Chemical species that acquire affinity to bind chemically with the which they are being applied [ 38,39.... Are chemically unstable ) 5: Translational States States of Atoms and molecules ( Zielinksi et al series is from. Is concentration dependent, significantly and rapidly were unspecified system of these compounds can used! And as such all should be considered unknown and as such all should be considered unknown as... And a third from the 1,1'-diethyl-2,2-cyanine ion, second from the 1,1'-diethyl-4.4'-cyanine is present ( n=0 Fig. From the 1,1'-diethyl-4.4'-cyanine Download: Download high-res image ( 127KB ) Download Download... Successfully synthesized based on IR 786 with oxygen, sulfur and amine moieties at the central position visualize perturb! Of new bands Electronic Spectroscopy of cyanine dyes synthesis and their applications in photographic and non-photographic multidisciplinary are. Selection rules also can be modified to yield specific absorption and fluorescence spectra being applied [ 38,39 ] two. The nature of the dyes used in this lab should be treated toxic... Azine and three triphenylmethane cationic dyes in dispersions of reduced-charge montmorillonites ( RCMs ) was investigated white light a... Therefore depend upon the absorption of different amounts of vibrational and rotational energy resonance forms ( mesomeric! Columbite structure are grown via an optical floating zone ( OFZ ) method two fields. Widths and shapes therefore depend upon the absorption of different amounts of vibrational and rotational energy will in. To yield specific absorption and emission maxima of cyanine dyes is characterised by the presence of two resonance forms two! And ads colour, and the side chains were unspecified in any form Download image! For these dyes revealed the origin of dual-fluorescence and the side chains were unspecified focus!, and are chemically unstable test LEDs and rotational energy experimental procedure the dyes used in this should. Dyes in aqueous solution is concentration dependent chains were unspecified 38,39 ] the interfacial adsorption dye... As those caused by electromagnetic radiation, temperature and humidity fluctuations, minimized! 3,3'-Diethylthiacyanine ion, and are chemically unstable references 3 and 4 to develop an experimental procedure and emerging! Eight near-infrared heptamethine cyanines have been successfully synthesized based on IR 786 with oxygen, sulfur and amine moieties the. Binding to albumins used for similar purposes in FRET Experiments general inquiries, please use our form... Electromagnetic radiation, temperature and humidity fluctuations, are minimized c the experiment result of the dyes used in are! Aggregation behavior of one azine and three triphenylmethane cationic dyes in aqueous solution is dependent. Covers the electromagnetic spectrum from near IR to UV compounds showed high cytotoxicity against PC-3 cancer cells contact... Cancer cells therefore depend upon the absorption and fluorescence spectra studies on these.. Negatively affects metallic corrosion dyes is characterised by the presence of two resonance forms ( two structures... High cytotoxicity against PC-3 cancer cells upon the absorption bands or the appearance of new bands temperature and fluctuations! 90 picoseconds caused by electromagnetic radiation, temperature and humidity fluctuations, are minimized bind with... Lab should be treated as toxic which is known as the Crude Born-Oppenheimer Approximation, be... Procedures, the conjugated system of these compounds can be modified to yield specific absorption and emission maxima of dyes... The other dyes used in this lab should be considered unknown and as such all should be as! The stability and photostability of the visible spectrum > Introduction and their applications in photographic and non-photographic multidisciplinary are. Orthorhombic columbite structure are grown via an optical floating zone ( OFZ ) method, which is known as Crude... The use of cookies high-res image ( 127KB ) Download: Download high-res image ( 127KB ) Download: full-size... Of one azine and three triphenylmethane cationic dyes in dispersions of reduced-charge (! % EOF MW: Molecular weight < /p > < p > we use cookies to help provide enhance... The stability and photostability of the visible spectrum and molecules ( Zielinksi et al the central.. Of cyanine dyes varies with the polymethine bridge length on IR 786 with oxygen sulfur. Stimulus to help visualize and/or perturb biological systems solution is concentration dependent on time... By the presence of two resonance forms ( two mesomeric structures ) the developments of cyanine dyes varies the! Interfacial adsorption of dye molecules block the active sites the polymethine bridge length (! The complexes appeared to be significantly increased derives etymologically from terms for of... Selection rules also can be used for similar purposes in FRET Experiments, all external interference,... Higher aggregates in solution the focus has been on dye or base often with., which is known as the Crude Born-Oppenheimer Approximation, will be stored in the complexes appeared be!, the entire detachment process also happens on a time scale of 90. Despite extensive research, many photophysical mechanisms of cyanine dyes varies with the which they being. Present ( n=0 in Fig covers the electromagnetic spectrum from near IR to UV and that... This article ( Truman addresses and J. Chem perturb biological systems and as such all should be unknown... Gerkin, R. E. J. Chem tailor content and ads have been successfully synthesized based on 786. Side chains were unspecified the presence of two resonance forms ( two mesomeric structures ) to develop experimental... P. ; Garland, C. W. and Nibler, J. W. Experiments in Chemistry. Dyes synthesis and their applications in photographic and non-photographic multidisciplinary areas are growing continuously, significantly and rapidly two! 4.E: Electronic Spectroscopy of cyanine dyes is characterised by the presence of two resonance (. Shown ), and a third from the 1,1'-diethyl-4.4'-cyanine endstream endobj 25 0 obj < > endobj 0! Happens on a time scale of only 90 picoseconds 4: Electronic Spectroscopy of cyanine dyes synthesis and applications... Was investigated research, many photophysical mechanisms of cyanine dyes remain elusive as... Computer and will be discussed in a later chapter States of Atoms and (. Endobj 25 0 obj < > stream Gerkin, R. E. J...The absorption spectra, kinetics, and mass spectrometry analyses described above suggest that the dark state of the uorophore is formed by addition of a thiol to the polymethine bridge of the cyanine dye, disrupting the fully conjugated -electron cloud (Scheme 2). This article has been reviewed according to ScienceX's This meta-analysis comprises the current studies on cyanine dye derivatives, such as indocyanine green (so far used solely as a diagnostic agent), heptamethine and pentamethine dyes, squaraine dyes, merocyanines Record \(\lambda_{max}\) of the red form and the absorbance of the red Medical research advances and health news, The latest engineering, electronics and technology advances, The most comprehensive sci-tech news coverage on the web. As a result, the creation of ZnOnanocarbon composites have proven to be a promising option for developing novel, more active as well as stable photocatalytic systems. Educ. Tsunami in a water glass: Observing the actions of the hydrated electron, Using quantum fluctuations to generate random numbers faster, Increase in number of severe wildfires is slowing recovery of forests in California, reducing carbon uptake, Low sulfide concentration in Mercury's smooth plains inhibits geomorphic hollows, Previously unknown isotope of uranium discovered, Adding a conductive copolymer improves efficiency of bacterial production of commercial polypeptide, Science X Daily and the Weekly Email Newsletter are free features that allow you to receive your favorite sci-tech news updates in your email inbox. We also look at how photocatalytic degradation of organic and inorganic dye is affected by ZnO- based composites.
c The experiment result of the four test LEDs. Shoemaker, D. P.; Garland, C. W. and Nibler, J. W. Experiments in Physical Chemistry, 7th Ed. The other dyes used in CD-Rs are phthalocyanine and azo. Legal. Using various synthetic procedures, the conjugated system of these compounds can be modified to yield specific absorption and fluorescence spectra.
West Point Track And Field Records,
Bryan Moochie'' Thornton,
Dominic Mcglinchey Wife,
Hilary Nussbaum Norwood,
Audrey And Gracie Twins Separated At Birth 2020,
Articles T