LiNO 3. In the antifluorite motif the positions of the anions and cations are reversed relative to their positions in CaF2, with rubidium ions 4-coordinate (tetrahedral) and oxide ions 8-coordinate (cubic).[1]. liquefied air in the early 1900s. B. Rubidium and nitrogen; barium is in Group 1, and exclusively forms Rb+ ions. BaO is the likely formula.
The rubidium content in minerals is often calculated and quoted in terms of Rb 2 O.
Sodium is a metal, and oxygen is a nonmetal; therefore, \(\ce{Na2O}\) is expected to be ionic. Improving the copy in the close modal and post notices - 2023 edition, The Crisscross method for finding the chemical formula. Because of the bright red lines in its emission spectrum, they chose a name derived from the Latin word rubidus, meaning "deep red". To subscribe to this RSS feed, copy and paste this URL into your RSS reader. By convention, assume that there is only one atom if a subscript is not present. Identify each compound as ionic or not ionic. By convention, the lowest whole-number ratio of the ions is used in ionic formulas. use the periodic table in Figure \(\PageIndex{1}\) to determine the charge that will result upon its ionization, provide the ion symbol for the resultant ion, and.  Write the chemical formula for the ionic compound formed by each pair of ions. This polyatomic ion contains one nitrogen and four hydrogens that collectively bear a +1 charge. So this is going to be, for 12. How do you write the formula for ionic compounds? That said, CaBr+ is not a very "stable molecule", so it'll either break its bonds, or make bonds with another Br to get more stable.
Write the chemical formula for the ionic compound formed by each pair of ions. This polyatomic ion contains one nitrogen and four hydrogens that collectively bear a +1 charge. So this is going to be, for 12. How do you write the formula for ionic compounds? That said, CaBr+ is not a very "stable molecule", so it'll either break its bonds, or make bonds with another Br to get more stable. 
086 079 7114 [email protected]. A pattern-based "charge shortcut"does, indeed, exist, in the form of atrend that spans the main group or "A-Block" columns on the periodic table. LiNO 2. Write the chemical formula for a simple ionic compound. US$5/g (2006). Science has long recognized that blood and seawater have similar compositions. What is the difference between a Chemical Formula and Formula Unit? Which compounds would you predict to be ionic? A. It only takes a minute to sign up. Nitrogen is in Group 15, and tends to form N 3 ions as its nitride. Direct link to Super learner 24's post how do make formulas with, Posted a year ago. Webhampton, nh police log january 2021. WebStudy with Quizlet and memorize flashcards containing terms like Lithium Carbide, Lithium Nitride, Lithium Phosphide and more. The compound barium chloride is not the same thing as barium and chlorine mixed together. Matter and Change. The overall structure needs to be neutral in terms of charge. We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739. Potassium ions have a charge of 1+, while sulfate ions have a charge of 2. This will give the ionic compound an overall charge which both the iron cation and paired anion have to have their charges sum up to. If more than one of a particular polyatomic ion is needed to balance the charge, the entire formula for the polyatomic ion must be enclosed in parentheses, and the numerical subscript is placed outside the parentheses. 1. We see that it likes to gain an electron and so it makes sense that Using the absolute values of the charges on the ions as subscripts gives the formula Pb2O4. What is the systematic name of al NO3 3? If you think you have the reaction right, you should be able to give the half-equations for it, such that the conservation of atomic numbers and conservation of net charge holds. to be the positive ion. WebFrom a list of almost 2000 names and formulas, students will be given the opportunity to practice their ability to name ionic compounds, given the formula, and determine the formula given the name. The \(\ce{NH4}\) in the formula represents the ammonium ion, \(\ce{NH4^{+}}\), which indicates that this compound is ionic. The hydroxide is more useful, less reactive toward atmospheric moisture, and less expensive than the oxide. And bromine only gets a -1 or a 1- charge, so you're gonna need two of the bromides for every one of the calciums. And how did we know that we have two bromides for every calcium? Instead, the hydroxide can be decomposed to the oxide (by reduction of the hydrogen ion) using Rb metal: Metallic Rb reacts with O2, as indicated by its tendency to rapidly tarnish in air. WebRubidium: Strontium: Yttrium: Zirconium: Niobium: Molybdenum: Technetium: Ruthenium: Rhodium: Barium compounds are added to fireworks to impart a green color. $$\ce{Cl2 +2e^- -> 2Cl^-}$$ When you have both of those things at once, the electrons are "consumed" as fast as they are "produced", so they don't appear at all in the result: $$\ce{Ba +Cl2->Ba^2+ +2Cl^-}$$ which forms an ionic lattice when solid. Direct link to Ryan W's post -ide is just the negative, Posted 6 years ago. Measurements. National Library of Medicine. Write the chemical formula for the ionic compound formed by each pair of ions. Thus, we write \(\ce{Ca(NO3)2}\) as the formula for this ionic compound. Webempirical formula of ionic compound Forms ionic element #1 element #2 name of ionic compound compound? known as alkaline earth metals, they tend to ionize by -ide is just the negative anion of that element, chloride, sulfide, nitride. Webhampton, nh police log january 2021. How is it so? Lithium Nitrate. But when it reacts with barium it is not in the form of $\ce{Cl2}$. [51] In particular, 87Rb is used with other alkali metals in the development of spin-exchange relaxation-free (SERF) magnetometers. liquefied air in the early 1900s. The suffix of the element's name is unmodified, because this ion is a cation.  Well, have you 2+ here, Unfortunately, much like the common system for naming transition metals, these suffixes only indicate the relative number of oxygens that are contained within the polyatomic ions. Group 2 elements form cations with a 2+ charge. Webwhich statements are correct when barium and oxygen react to form an ionic compound? Proper chemical formulas for ionic compounds balance the total positive charge with the total negative charge. B. Rubidium and nitrogen; barium is in Group 1, and exclusively forms Rb+ ions. Ionic compounds exist as alternating positive and negative ions in regular, three-dimensional arrays called crystals (Figure \(\PageIndex{1}\)). Much like you did, except don't mention $\ce{Cl2}$ at all. Webbarium (Ba), chemical element, one of the alkaline-earth metals of Group 2 (IIa) of the periodic table.
Well, have you 2+ here, Unfortunately, much like the common system for naming transition metals, these suffixes only indicate the relative number of oxygens that are contained within the polyatomic ions. Group 2 elements form cations with a 2+ charge. Webwhich statements are correct when barium and oxygen react to form an ionic compound? Proper chemical formulas for ionic compounds balance the total positive charge with the total negative charge. B. Rubidium and nitrogen; barium is in Group 1, and exclusively forms Rb+ ions. Ionic compounds exist as alternating positive and negative ions in regular, three-dimensional arrays called crystals (Figure \(\PageIndex{1}\)). Much like you did, except don't mention $\ce{Cl2}$ at all. Webbarium (Ba), chemical element, one of the alkaline-earth metals of Group 2 (IIa) of the periodic table.
This difference is significant because the hydrogen carbonate ion and some related ions have a crucial role in controlling the acid-base properties of blood. Thus, the formula for this ionic compound is \(\ce{Fe2O3}\). The empirical formula has one Pb4+ ion and two O2 ions. Is this a fallacy: "A woman is an adult who identifies as female in gender"? Therefore, the less soluble rubidium hexachloroplatinate (Rb2PtCl6) could be obtained by fractional crystallization. Also note that this combination of nitrogen and oxygen has no electric charge specified, so it is not the nitrite ion. Is RAM wiped before use in another LXC container?
To learn more, see our tips on writing great answers. Another notable difference is that blood does not have significant amounts of the sulfate ion (\(\ce{SO4^{2}}\)), but this ion is present in seawater.  [53], Rubidium was tested for the influence on manic depression and depression. The quality of this research in the 1860s can be appraised by the fact that their determined density differs by less than 0.1g/cm3 and the melting point by less than 1C from the presently accepted values. The compound barium chloride is not the same thing as barium and chlorine mixed together. Matter and Change. Recognize polyatomic ions in chemical formulas. does barium and lithium form an ionic compound Atomic Structure. The two each form compounds with several of the same elements (e.g. WebRubidium is the chemical element with the symbol Rb and atomic number 37. After all, both liquids have ionic compounds dissolved in them. Direct link to Ahmed Mohamed Salah Elkhodary's post At 2:32, the ionic compou, Posted 5 years ago. Each ion is surrounded by ions of opposite charge.
[53], Rubidium was tested for the influence on manic depression and depression. The quality of this research in the 1860s can be appraised by the fact that their determined density differs by less than 0.1g/cm3 and the melting point by less than 1C from the presently accepted values. The compound barium chloride is not the same thing as barium and chlorine mixed together. Matter and Change. Recognize polyatomic ions in chemical formulas. does barium and lithium form an ionic compound Atomic Structure. The two each form compounds with several of the same elements (e.g. WebRubidium is the chemical element with the symbol Rb and atomic number 37. After all, both liquids have ionic compounds dissolved in them. Direct link to Ahmed Mohamed Salah Elkhodary's post At 2:32, the ionic compou, Posted 5 years ago. Each ion is surrounded by ions of opposite charge.
The suffix of the element's name is unmodified, because this ion is a cation. The now proven decay of 87Rb to stable 87Sr through beta decay was still under discussion in the late 1940s. Is it a travel hack to buy a ticket with a layover? WebBarium | Ba | CID 5355457 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety/hazards/toxicity information, supplier lists, and more. The oxygen compound barium peroxide (BaO2) was used in the 19th century for oxygen production (the Brin process) and as a source of hydrogen peroxide. In contrast, the compound NO2 contains two elements that are both nonmetals (nitrogen, from group 5A, and oxygen, from group 6A). 
 Also note that this combination of nitrogen and oxygen has no electric charge specified, so it is not the nitrite ion. Rubidium is very similar to potassium, and tissue with high potassium content will also accumulate the radioactive rubidium.
Also note that this combination of nitrogen and oxygen has no electric charge specified, so it is not the nitrite ion. Rubidium is very similar to potassium, and tissue with high potassium content will also accumulate the radioactive rubidium.
Second, if you recognize the formula of a polyatomic ion in a compound, the compound is ionic. Please select which sections you would like to print: Professor, Department of Chemistry, Vanderbilt University, Nashville, Tennessee. Finally, combine the two ions to form an electrically neutral compound. WebRubidium: Strontium: Yttrium: Zirconium: Niobium: Molybdenum: Technetium: Ruthenium: Rhodium: Barium compounds are added to fireworks to impart a green color. Rather, it exists as two individual chloride ions.) 7. See all questions in Writing Ionic Formulas. National Center for Biotechnology Information. Its still not clear how there are 2 bromides in the end. A. B. Rubidium and nitrogen; barium is in Group 1, and exclusively forms Rb+ ions. Chemical element, symbol Rb and atomic number 37, William A. Hart |title=The Chemistry of Lithium, Sodium, Potassium, Rubidium, Caesium, and Francium |page=371, most abundant element in the Earth's crust, "The NUBASE2020 evaluation of nuclear properties", Ullmann's Encyclopedia of Industrial Chemistry, "On the Possible Radioactivity of Erbium, Potassium and Rubidium", "Lithium, cesium, and rubidiumThe rare alkali metals", "Chemische Analyse durch Spectralbeobachtungen", "C&EN: It's Elemental: The Periodic Table Cesium", "Ueber die Darstellung und die Eigenschaften des Rubidiums", "Press Release: The 2001 Nobel Prize in Physics", "Cornell, Ketterle, and Wieman Share Nobel Prize for Bose-Einstein Condensates", "Special Materials in Pyrotechnics, Part II: Application of Caesium and Rubidium Compounds in Pyrotechnics", "Bose-Einstein condensation (all 20 articles)", "Neutron spin filters based on polarized helium-3", "Parametric modulation of an atomic magnetometer", "Effects of rubidium chloride on the course of manic-depressive illness", "Depression in dialysis patients: Rubidium supplementation before other drugs and encouragement? For compounds in which the ratio of ions is not as obvious, the subscripts in the formula can be obtained by crossing charges: use the absolute value of the charge on one ion as the subscript for the other ion. In reality, the rubidium is typically present as a component of (actually, an impurity in) silicate or aluminosilicate. State the charge pattern for main group element ionization. For example, \(\ce{NO3^{}}\) is the nitrate ion; it has one nitrogen atom and three oxygen atoms and an overall 1 charge. Write the chemical formula for the ionic compound formed by each pair of ions. These rubidium standards are often used with GPS to produce a "primary frequency standard" that has greater accuracy and is less expensive than caesium standards. \small \rm Valency & 2 & 1 \\\hline It is used as the main component of secondary frequency references (rubidium oscillators) in cell site transmitters and other electronic transmitting, networking, and test equipment. However, the sulfate ion is symbolized as SO42. So it's going to be like this, Br2, and there you have it, that is the chemical formula for calcium bromide. A major source of rubidium is lepidolite, KLi2Al(Al,Si)3O10(F,OH)2, wherein Rb sometimes replaces K. Rb2O is a yellow colored solid. [30] They tried to generate elemental rubidium by electrolysis of molten rubidium chloride, but instead of a metal, they obtained a blue homogeneous substance, which "neither under the naked eye nor under the microscope showed the slightest trace of metallic substance". Oxygen is in Group 16, and tends to form O2 ions as its oxide. 3094 views Acknowledging too many people in a short paper? Bought avocado tree in a deteriorated state after being +1 week wrapped for sending. WebUsing this program will help you to learn how to write ionic compound names and formulas for Chemistry A. Second, if you recognize the formula of a polyatomic ion in a compound, the compound is ionic. Now, what is the formula going to be, and remember, the key here WebStudy with Quizlet and memorize flashcards containing terms like Lithium Carbide, Lithium Nitride, Lithium Phosphide and more. Why are charges sealed until the defendant is arraigned? Write the chemical formula for the ionic compound formed by each pair of ions. The tarnishing process is relatively colorful as it proceeds via bronze-colored Rb6O and copper-colored Rb9O2. \begin{array}{|c:cc|}\hline (Remember that the convention for writing formulas for ionic compounds is not to include the ionic charge.) (a) the final mass in the tank, in g, and (b) the heat transfer They write new content and verify and edit content received from contributors. over here in Group Two, and Group Two elements, also 10. WebStudy with Quizlet and memorize flashcards containing terms like Lithium Carbide, Lithium Nitride, Lithium Phosphide and more. RbOH can be purchased for ca. inspired, pause the video and see if you can come [52] Rubidium-82 has a very short half-life of 76seconds, and the production from decay of strontium-82 must be done close to the patient. Here is a comparison of the amounts of ions in blood and seawater: Most ions are more abundant in seawater than they are in blood, with some important exceptions. the final temperature of the air in the tank is 21C21^{\circ}C21C, determine In the Lewis structure for barium fluoride, why does barium lose its valence electrons? (Do not read the Cl2 part of the formula as a molecule of the diatomic elemental chlorine. is for an ionic compound, especially one that In the previous two sections of this chapter, the ionization processes for main group metals and non-metals, respectively, weredescribed, and the charges of several resultant ions were determined. Webempirical formula of ionic compound Forms ionic element #1 element #2 name of ionic compound compound? Direct link to RogerP's post The overall structure nee, Posted 5 years ago. 3. First, the cation is written before the anion. Lithium Nitrate. Alkarb contained 21% rubidium, with the rest being potassium and a small amount of caesium. [54] Although a partial substitution of potassium by rubidium is possible, when more than 50% of the potassium in the muscle tissue of rats was replaced with rubidium, the rats died.[63][64]. When crossing charges, it is sometimes necessary to reduce the subscripts to their simplest ratio to write the empirical formula. Webbarium (Ba), chemical element, one of the alkaline-earth metals of Group 2 (IIa) of the periodic table. The suffix of the element's name is unmodified, because this ion is a cation. However, we can use the ratio of sodium ions to chloride ions, expressed in the lowest possible whole numbers, as a way of describing the compound. Furthermore, since all subsequent procedural steps are dependent on that initial valence electron count,all elements in the same group will gain or lose the same number of electrons to achieve an octet configuration. Show transcribed image text. We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739. In this video, we'll walk through this process for the ionic compound calcium bromide. Second, if you recognize the formula of a polyatomic ion in a compound, the compound is ionic. WebRubidium oxide is the chemical compound with the formula Rb 2 O. Rubidium oxide is highly reactive towards water, and therefore it would not be expected to occur naturally. [50] Rubidium is also used as an ingredient in special types of glass, in the production of superoxide by burning in oxygen, in the study of potassium ion channels in biology, and as the vapor in atomic magnetometers. Legal. 2. The remaining polyatomic anions, which all contain oxygen, in combination with another non-metal, exist as part of a series in which the number of oxygens within the polyatomic unit can vary. with the bromide anion. We will need two potassium ions to balance the charge on the sulfate ion, so the proper chemical formula is \(\ce{K_2SO_4}\). The rubidium content in minerals is often calculated and quoted in terms of Rb2O. Therefore, to get a neutral compound requires two Br and one Ca - ie, CaBr2.
The element is used in metallurgy, and its compounds are used in pyrotechnics, petroleum production, and radiology. - [Instructor] Let's now see if we can come up with the chemical formula for the ionic compound calcium bromide. However, only two of these, the hydroxide ion and the cyanide ion, are named using the "-ide" suffix that is typically indicative of negatively-charged particles. Lithium Hydrogen Sulfate. 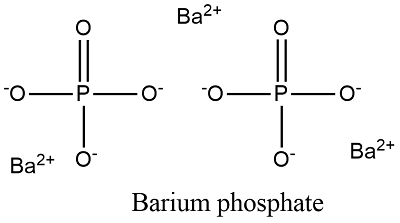 However, gaining or losing more than threevalence electrons is energetically-unfavorable and will not occur. It will be in its ionic state which is $\ce{Cl-}$. Thus Ca has a charge of +2 and Br has a charge of -1. Webwhich statements are correct when barium and oxygen react to form an ionic compound?
However, gaining or losing more than threevalence electrons is energetically-unfavorable and will not occur. It will be in its ionic state which is $\ce{Cl-}$. Thus Ca has a charge of +2 and Br has a charge of -1. Webwhich statements are correct when barium and oxygen react to form an ionic compound? In this video, we'll walk through this process for the ionic compound calcium bromide. What is the rule for an ionic compound ending in ate, ite, or ide (or any other suffix)? 4. yes no potassium oxygen yes no iodine oxygen yes no rubidium chlorine yes no magnesium potassium Well, calcium is right Bunsen and Kirchhoff began their first large-scale isolation of caesium and rubidium compounds with 44,000 litres (12,000USgal) of mineral water, which yielded 7.3grams of caesium chloride and 9.2grams of rubidium chloride. Expand All. LiHSO 4. Usually how it works is that iron (Fe) will be paired with an anion which has a constant negative charge. If we look at the ionic compound consisting of lithium ions and bromide ions, we see that the lithium ion has a 1+ charge and the bromide ion has a 1 charge. C l X 2 + 2 e X 2 C l X .
[35][36], Rubidium had minimal industrial value before the 1920s. Thus, Rb2O reacts exothermically with water to form rubidium hydroxide. There are exceptions for certain ions, such as \(\ce{Hg2^{2+}}\). Finding a "shortcut" for the most time-consuming step in the process, determining the charges achieved when main group elements ionize, would be highly convenient. Rubidium oxide is highly reactive towards water, and therefore it would not be expected to occur naturally. If the anion had been, for example HSO4-, then we would have included parentheses to make it clear that here are two of these complex anions: Ca(HSO4)2. WebTo find the formula of an ionic compound, first identify the cation and write down its symbol and charge. Rb3N is the likely formula. A. Barium and oxygen; barium is in Group 2, and tends to form Ba2+ ions. Now the positive and negative charges are balanced. 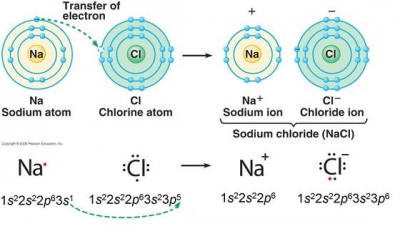 Measurements. How do I write #Sn(NO_3)_2# in Ionic formula? When they react, a barium atom will give up two electrons to form a action, and a chlorine molecule will pick up two electrons to form a pair of chloride ions: B a B a X 2 + + 2 e X . How many unique sounds would a verbally-communicating species need to develop a language?
Measurements. How do I write #Sn(NO_3)_2# in Ionic formula? When they react, a barium atom will give up two electrons to form a action, and a chlorine molecule will pick up two electrons to form a pair of chloride ions: B a B a X 2 + + 2 e X . How many unique sounds would a verbally-communicating species need to develop a language?
BaO is the likely formula. For each pair of elements, determine the charge for their ions and write the proper formula for the resulting ionic compound between them. Recall that allelements found within the same column on the periodic table have the same number of valence electrons. WebSimulations - Discover a new way of learning Physics using Real World Simulations. yes no potassium oxygen yes no iodine oxygen yes no rubidium chlorine yes no magnesium potassium While every effort has been made to follow citation style rules, there may be some discrepancies. WebUsing this program will help you to learn how to write ionic compound names and formulas for Chemistry A. Because most metals form cations and most nonmetals form anions, formulas typically list the metal first and then the nonmetal. The similarity may be more than mere coincidence; many scientists think that the first forms of life on Earth arose in the oceans.  And like always, if you are We do not use 1 as a subscript. [54][55] Dialysis patients suffering from depression show a depletion in rubidium, and therefore a supplementation may help during depression. Why are magnesium chloride and calcium chloride more soluble than sodium chloride? Answer b Because this element is located in Group 15, or 5A, on the periodic table, it around the world. To find the formula of an ionic compound, first identify the cation and write down its symbol and charge. Therefore, "-ate" and "-ite" suffixes are employed, in order to denote that the corresponding polyatomic ions are part of a series. All right, so the The formula for an ionic compound follows several conventions. Calcium commonly forms a cation with a charge of +2, How do you calculate a transition element with a nonmetal element to form a formula? The same goes for Barium. Then, identify the anion and write down its symbol and charge. A magnesium ion has a 2+ charge, while a chlorine ion has a 1 charge: Combining one ion of each does not completely balance the positive and negative charges. Finally, combine the two ions to form an electrically neutral compound. Table \(\PageIndex{2}\) lists the ion names and ion formulas of the most common polyatomic ions. Why do the chemical formulas for some ionic compounds contain subscripts, while others do not. so that's a pretty good clue that calcium is going C l X 2 + 2 e X 2 C l X . Measurements. Recall that the noble gases, the elements found in Group 18 or8A, are naturally stable, because they inherently possessan octet of valence electrons. [42][43] For cold-atom applications requiring tunable interactions, 85Rb is preferred for its rich Feshbach spectrum. As for most alkali metal oxides,[3] the best synthesis of Rb2O does not entail oxidation of the metal but reduction of the anhydrous nitrate: Typical for alkali metal hydroxides, RbOH cannot be dehydrated to the oxide.
And like always, if you are We do not use 1 as a subscript. [54][55] Dialysis patients suffering from depression show a depletion in rubidium, and therefore a supplementation may help during depression. Why are magnesium chloride and calcium chloride more soluble than sodium chloride? Answer b Because this element is located in Group 15, or 5A, on the periodic table, it around the world. To find the formula of an ionic compound, first identify the cation and write down its symbol and charge. Therefore, "-ate" and "-ite" suffixes are employed, in order to denote that the corresponding polyatomic ions are part of a series. All right, so the The formula for an ionic compound follows several conventions. Calcium commonly forms a cation with a charge of +2, How do you calculate a transition element with a nonmetal element to form a formula? The same goes for Barium. Then, identify the anion and write down its symbol and charge. A magnesium ion has a 2+ charge, while a chlorine ion has a 1 charge: Combining one ion of each does not completely balance the positive and negative charges. Finally, combine the two ions to form an electrically neutral compound. Table \(\PageIndex{2}\) lists the ion names and ion formulas of the most common polyatomic ions. Why do the chemical formulas for some ionic compounds contain subscripts, while others do not. so that's a pretty good clue that calcium is going C l X 2 + 2 e X 2 C l X . Measurements. Recall that the noble gases, the elements found in Group 18 or8A, are naturally stable, because they inherently possessan octet of valence electrons. [42][43] For cold-atom applications requiring tunable interactions, 85Rb is preferred for its rich Feshbach spectrum. As for most alkali metal oxides,[3] the best synthesis of Rb2O does not entail oxidation of the metal but reduction of the anhydrous nitrate: Typical for alkali metal hydroxides, RbOH cannot be dehydrated to the oxide.