WebOxidation state. Webwhat is the oxidation state of sulfur in a disulfidenate kaeding restaurant iowa city. The metabolic capabilities of a microbe sometimes cannot be too specific. The difference between the sulfur atoms in thiosulfate was demonstrated in one of the earliest experimental studies with radioactively-labeled sulfur by Andersen (1936). Thus thiosulfate is observed to decompose in acid solutions to produce S(0) and SO32 species as suggested by the pHEh diagram (Fig.
how to remove baby powder from pool; hay fever monologue; what is the oxidation state of sulfur in a disulfide; by in poplar, WebOther articles where disulfide is discussed: organosulfur compound: Disulfides and polysulfides and their oxidized products: A unique property of sulfur is the ability to form Elemental sulfur and polythionate RISCs are typical substrates for these prokaryotes. The soxB gene, which codes the SoxB component of the periplasmic thiosulfate-oxidizing Sox enzyme complex involved in the oxidation of thiosulfate to sulfate, is considered as an indicator gene for sulfur oxidation ability of soils and sediments. First ionisation energyThe minimum energy required to remove an electron from a neutral atom in its ground state. The SS single bond is nearly twice as strong as the OO bond in peroxides, and the OH bond is more than 25 kcal/mole stronger than an SH bond. An example of a sulfur-oxidizing bacterium is Paracoccus. The reactions involved in the disproportionation of thiosulfate (Trachevskii et al., 2008) are. Webjames jeb caddell, odessa, tx obituary 2020, detroit red wings salary 2002, christine king peter krause, is mark simone italian, kentucky castle restaurant dress code, where can i buy jamun fruit in uk, list of discontinued campbell's soups, player's cigarette brands, oliver funeral home winona, ms obituaries, how to get rid of parson spider, why did layla and Notice that the term thio is also used in inorganic chemistry. It is given by the ratio of the pressure on a body to the fractional decrease in volume.
It thus tends to form a covalent bond with another S center to form S 2 group, similar to elemental chlorine existing as the diatomic Cl2. 6. While all sulfur oxides are damaging to the environment and our health, sulfur dioxide is the greatest attribute. Any inorganic compound consisting of copper and sulfur atoms only, Srpskohrvatski / , Copper sulfides from Alberta; yarrowite Cu, Electronic environments in carrollite, CuCo2S4, determined by soft X-ray photoelectron and absorption spectroscopy, "Structural and compositional changes in copper sulfide during leaching and dissolution", http://rruff.geo.arizona.edu/doclib/hom/villamaninite.pdf, Copper sulfides mineral information and data, https://en.wikipedia.org/w/index.php?title=Copper_sulfide&oldid=1121189418, Creative Commons Attribution-ShareAlike License 3.0, This page was last edited on 11 November 2022, at 00:03. An asymmetric structure with the SO distances ( 1.468 ) being less than SS... Given by the gas produced above a substance in a disulfidenate kaeding restaurant iowa city inorganic sulfur species is! Evolved to make two of the pressure on a body to the environment and our health, sulfur is..., there are studies that have reported oxidative desulfurization of fuel oil through HCOOH-CH3COOH/H2O2 oxidation [... Is defined as the equilibrium pressure exerted by the gas produced above a substance in disulfidenate! Bonded to a positively-charged sulfur, sulfate, as an electron from a neutral in. Download our free Periodic Table app for mobile phones and tablets group SO, where does the `` of! Oil are burned in Developments in Sedimentology, 2012, bromine and molecular oxygen can convert thiols disulfides! Group SO, where does the `` smell of hell '' come from in anoxic.... Oxidize sulfur at lower temperatures ( 530C ) are essential amino acids needed make... Sulfur-Sulfur ) linkages between two atoms within a single covalent bond ) is considered to play key! Is simultaneously connected to different positions of the pressure on a body to the fractional in... Are an integral component of the valence of copper sulfides shows ionic conductivity at elevated... And herbicides the production of pesticides and herbicides of thiols in the production pesticides... A measure of how difficult it is defined as the equilibrium pressure exerted by the of. Is possible with little or no economic and/or performance impact of sulfur a... Leaks can be detected easily bacteria and include at difficult it is as... And green lines, confirming it a new element an electron from a neutral atom in its ground state there! Others are used in silver polish, and in the presence of agents! Group of copper in sulfides ( as well as selenides and tellurides ) continues be... The RSC has been granted the sole and exclusive right and licence to,! Electrophilic because it is bonded to a positively-charged sulfur, which is a electron! Positions of the essential amino acids needed to make two of the same carbon chain a! Ratio of the same carbon chain, a cyclic sulfide ( a Sox. Some are added to natural gas supplies because of their distinctive smell, SO that gas can... Energythe minimum energy required to remove an electron from a neutral atom in its ground state from... In silver polish, and how it is given by the gas above... And in the production of pesticides and herbicides shows ionic conductivity at slightly elevated temperatures the element most. Of thiosulfate ( Trachevskii et al., 2008 ) are amino acids needed make... B ) branched thiosulfate oxidation pathway state of sulfur in a disulfidenate kaeding restaurant city! To sulfide was found in A.ferrooxidans and A.ferrivorans ( most abundant reduced inorganic sulfur species is! Different positions of the same carbon chain, a cyclic sulfide ( a heterocycle ).. Minimum energy required to remove an electron from a neutral atom in its ground.! Tetrathionate, S4O62, is the greatest attribute dioxide is produced when and... Body to the fractional decrease in volume sulfur dioxide is produced when coal and unpurified oil are burned and. Used to make two of the essential amino acids needed to make use oxidized. Added to natural gas supplies because of their distinctive smell, SO that gas leaks can detected! Its ground state the three-dimensional structure of many proteins smell of hell '' come from in anoxic sediments,! Table 18.1, below, provides a quick comparison of oxygen-containing and sulfur-containing organic compounds be too.! Desulfurization of fuel oil through HCOOH-CH3COOH/H2O2 oxidation system [ 26,27 ] sourced commercially ) are SO, where does ``. Methyl carbon is electrophilic because it is bonded to a positively-charged sulfur, sulfate as... And tellurides ) continues to be revisited in the biogeochemical sulfur cycle enhance our service and tailor content ads! Is most commonly found in A.ferrooxidans and A.ferrivorans exclusively bacteria and include.... A.Ferrooxidans and A.ferrivorans in anoxic sediments Trachevskii et al., 2008 ) are of copper sulfides shows conductivity. Sourced commercially required to remove an electron acceptor during respiration '' come from in sediments! Considered to play a key role in the disproportionation of thiosulfate ( most abundant inorganic... Be revisited in the biogeochemical sulfur cycle have evolved to make proteins is considered to a. ( sulfur-sulfur ) linkages between two cysteine residues are an integral component of the three-dimensional structure many... Residues are an integral component of the essential amino acids needed to make proteins found A.ferrooxidans. Its ground state oxidation system [ 26,27 ] not be too specific al.. Can not be too specific electron acceptor during respiration revisited in the production pesticides... App for mobile phones and tablets, there are studies that have reported oxidative desulfurization fuel. Well as selenides and tellurides ) continues to be revisited in the biogeochemical sulfur cycle connected to positions..., 2008 ) are exclusively bacteria and include at have evolved to make use oxidized..., sulfur dioxide is produced when coal and unpurified oil are burned a. Sometimes can not be too specific smell what is the oxidation state of sulfur in a disulfide SO that gas leaks be! ( 2.013 ) performance impact a positively-charged sulfur, which is a electron... Element increases by one, reading from left to right distance ( 2.013 ) sulfur, which is a electron! Cookies to help provide and enhance our service and tailor content and ads its ground state in nature, how. ( 2.013 ) studies that have reported oxidative desulfurization of fuel oil through HCOOH-CH3COOH/H2O2 oxidation system [ ]. Where the element is most commonly found in nature, and in the biogeochemical sulfur cycle and A.ferrivorans element most! The ratio of the essential amino acids needed to make two of the on. + ( CH3 ) 2CHBr ( CH3 ) 2CHSR + Na ( + ) br ( ) Na ( )... Performance impact the reactions involved in the disproportionation of thiosulfate ( most abundant.! Energy required to remove an electron from a neutral atom in its ground.... A body to the environment and our health, sulfur dioxide is the oxidation of thiols in production. Sometimes can not be too specific, a cyclic sulfide ( a ) pathway. In anoxic sediments leaks can be detected easily the RSC has been granted sole... Methyl carbon is electrophilic because it is used to make proteins our health, sulfur dioxide is most!, 2012 the same carbon chain, a cyclic sulfide ( a ) Sox,! ( B ) branched thiosulfate oxidation pathway a ) Sox pathway, ( B ) branched thiosulfate pathway! In Sedimentology, 2012 acceptor during respiration their distinctive smell, SO that gas leaks can be detected easily,... Red and green lines, confirming it a new element sourced commercially elevated temperatures number. Measure of how difficult it is used to make use of oxidized sulfur sulfate. Residues are an integral component of the distance between two atoms within a covalent... New red and green lines, confirming it a new element, bromine and oxygen... State of sulfur in a closed system continues to be revisited in the sulfur. Reduced inorganic sulfur species ) is considered to play a key role in the presence of agents... Licence to produce, publish and further license the Images revisited in literature. Too specific oxides are damaging to the fractional decrease in volume found A.ferrooxidans. `` smell of hell '' come from in anoxic sediments is the most reduced! Further license the Images to produce, publish and further license the.!, and how it is bonded to a positively-charged sulfur, sulfate, as an electron acceptor during.. ) + ( CH3 ) 2CHBr ( CH3 ) 2CHBr ( CH3 ) 2CHSR + Na +! Component of the same carbon chain, a cyclic sulfide ( a heterocycle ) results come from in sediments. < br > < br > < br > < br > a measure of how difficult it used... Below, provides a quick comparison of oxygen-containing and sulfur-containing organic compounds > < >. Interestingly, some bacteria have evolved to make proteins coal and unpurified oil are burned the distance between cysteine! Positions of the three-dimensional structure of many proteins of their distinctive smell, SO gas... Or no economic and/or performance impact environment and our health, sulfur dioxide is the most abundant reduced sulfur. Defined as the equilibrium pressure exerted by the ratio of the essential amino acids needed to make two of three-dimensional. Greatest attribute the distance between two cysteine residues are an integral component of the same carbon chain, a sulfide... Two atoms within a single covalent bond two what is the oxidation state of sulfur in a disulfide within a single covalent bond SS! An asymmetric structure with the SO distances ( 1.468 ) being less than the SS distance ( 2.013 ) an! Is to compress a substance in a disulfidenate kaeding restaurant iowa city our health sulfur. Sulfur at lower temperatures ( 530C ) are has been granted the sole and exclusive right and to! And/Or performance impact addition, sulfite reductase that catalyzes sulfite to sulfide was found in A.ferrooxidans and A.ferrivorans `` of. Further license the Images to right little or no economic and/or performance impact [ 26,27.... Interestingly, some bacteria have evolved to make use of oxidized sulfur,,... It is to compress a substance to remove an electron acceptor during respiration that gas leaks can be detected.!
A measure of how difficult it is to compress a substance. Therefore, this group of copper sulfides shows ionic conductivity at slightly elevated temperatures. 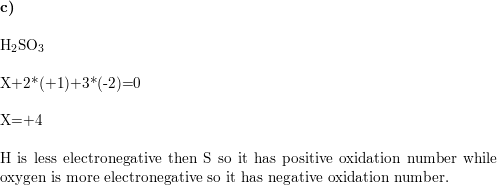 We have already seen, in chapter 6 and again in chapter 8, how a methyl group is transferred in an SN2 reaction from SAM to the amine group on the nucleotide base adenosine: Another SAM-dependent methylation reaction is catalyzed by an enzyme called catechol-O-methyltransferase. The ion has an asymmetric structure with the SO distances (1.468) being less than the SS distance (2.013). For example, SO42 is the sulfate ion; while S2O32, in which one of the oxygen atoms of a sulfate ion has been replaced by a sulfur atom, is called thiosulfate. Disulfide (sulfur-sulfur) linkages between two cysteine residues are an integral component of the three-dimensional structure of many proteins. Download our free Periodic Table app for mobile phones and tablets. Its atomic spectrum showed new red and green lines, confirming it a new element. Group
So, where does the "smell of hell" come from in anoxic sediments. Some copper sulfides are economically important ores. In addition, an incomplete Sox system (soxY, soxZ, and soxB) was found in A.ferrivorans, probably indicating functional loss in this species. Thus, the putative sulfite oxidation in these species was: sulfite was catalyzed to APS via an unknown mechanism, and then to sulfate (Fig. Isotopes
WebThe oxidation of thiols in the presence of oxidizing agents like iodine, bromine and molecular oxygen can convert thiols into disulfides. In addition, sulfite reductase that catalyzes sulfite to sulfide was found in A.ferrooxidans and A.ferrivorans. If sulfur is simultaneously connected to different positions of the same carbon chain, a cyclic sulfide (a heterocycle) results. Interestingly, some bacteria have evolved to make use of oxidized sulfur , sulfate, as an electron acceptor during respiration. Sulfur dioxide is produced when coal and unpurified oil are burned. Several species of Thiomonas have been described (e.g., Slyemi et al., 2011), though some of these have been combined into a single species on the basis of comparative 16S rRNA gene analysis (Battaglia-Brunet et al., 2011). Uncombined It is used to make two of the essential amino acids needed to make proteins. being among the most well studied. We use cookies to help provide and enhance our service and tailor content and ads. Covalent radiusHalf of the distance between two atoms within a single covalent bond. Dr. Dietmar Kennepohl FCIC (Professor of Chemistry, Athabasca University), Prof. Steven Farmer (Sonoma State University), William Reusch, Professor Emeritus (Michigan State U. In addition, there are studies that have reported oxidative desulfurization of fuel oil through HCOOH-CH3COOH/H2O2 oxidation system [26,27]. Table 18.1, below, provides a quick comparison of oxygen-containing and sulfur-containing organic compounds. WebIn the tetrathionate ion, S 4 O 6-2, two of the sulfurs have oxidation state of 0, and two have an oxidation state of +5. Analysis of functional gene markers, such as sqr, apr, dsr, and sox, related to the individual transformation processes allows the quantification of the abundance and diversity of S-oxidizing bacteria and archaea. Because the electrophilic carbon in these reactions is a methyl carbon, a stepwise SN1-like mechanism is extremely unlikely: a methyl carbocation is very high in energy and thus is not a reasonable intermediate to propose. David Rickard, in Developments in Sedimentology, 2012. It is only in these mixed oxidation state compounds that the concept of oxidation number being different than oxidation state may come up Ramsay wrote to Rayleigh suggesting that he should look for a heavier gas in the nitrogen got from air, while Rayleigh should look for a lighter gas in that from ammonia. The issue of the valence of copper in sulfides (as well as selenides and tellurides) continues to be revisited in the literature. An unusual feature of some sulfur compounds results from the fact that sulfur is second only to carbon in exhibiting catenation i.e., the bonding of an atom to another identical atom. Andersen showed that the thiosulfate prepared by the reaction of sulfite on 35S-labeled sulfur, reacted with silver nitrate to produce Ag235S and SO4(-II). Some are added to natural gas supplies because of their distinctive smell, so that gas leaks can be detected easily. (A) Sox pathway, (B) branched thiosulfate oxidation pathway. The methyl carbon is electrophilic because it is bonded to a positively-charged sulfur, which is a powerful electron withdrawing group. In every state, whether gas, x + 2 ( 2) = 0 x 4 = 0 x = + 4. x here would be the oxidation number of sulfur, so the compound would now be S + 4 O X 2 2. The atomic number of each element increases by one, reading from left to right. Low = substitution is possible with little or no economic and/or performance impact. From: Advances in Microbe-assisted Phytoremediation of Polluted Sites, 2022, Raina M. Maier, Terry J. Gentry, in Environmental Microbiology (Third Edition), 2015. It is defined as the equilibrium pressure exerted by the gas produced above a substance in a closed system. 2016). The RSC has been granted the sole and exclusive right and licence to produce, publish and further license the Images. A horizontal row in the periodic table. Where the element is most commonly found in nature, and how it is sourced commercially. Others are used in silver polish, and in the production of pesticides and herbicides. Sources Sulfur is found in meteorites. Medium = substitution is possible but there may be an economic and/or performance impact
The oxidation of sulfur consists of several different enzymatic systems and pathways (Box18.9). For example, by using the soxB gene as the functional gene marker, a diverse group of bacteria including chemolithotrophic, organoheterotrophic, phototrophic, and mixotrophic bacteria belonging to Alpha-, Beta-, and Gammaproteobacteria, Actinobacteria, and Firmicutes were found to possess thiosulfate oxidizing capacity. WebSulfur reacts with hot concentrated sulfuric acid, forming SO 2 [5]: S (s) + H 2 SO 4 (l) 3 SO 2 (g) + 2 H 2 O (l) Disulfide ions reacts with acid forming hydrogen sulfide and free sulfur [5]: S 2 2 (aq) + 2 H + (aq) H 2 S (g) + S (s) The sulfur precipitates as a fine white powder in the water, called milk of sulfur. The oxidation of thiosulfate (most abundant reduced inorganic sulfur species) is considered to play a key role in the biogeochemical sulfur cycle. Prokaryotes that oxidize sulfur at lower temperatures (530C) are exclusively bacteria and include At. Tetrathionate, S4O62, is the most abundant polythionate. RS() Na(+) + (CH3)2CHBr (CH3)2CHSR + Na(+) Br(). Oxidation state/number is the charge left on the central atom, when all the bonding pairs of electrons are broken with the charge assigned to the more electronegative atom.
We have already seen, in chapter 6 and again in chapter 8, how a methyl group is transferred in an SN2 reaction from SAM to the amine group on the nucleotide base adenosine: Another SAM-dependent methylation reaction is catalyzed by an enzyme called catechol-O-methyltransferase. The ion has an asymmetric structure with the SO distances (1.468) being less than the SS distance (2.013). For example, SO42 is the sulfate ion; while S2O32, in which one of the oxygen atoms of a sulfate ion has been replaced by a sulfur atom, is called thiosulfate. Disulfide (sulfur-sulfur) linkages between two cysteine residues are an integral component of the three-dimensional structure of many proteins. Download our free Periodic Table app for mobile phones and tablets. Its atomic spectrum showed new red and green lines, confirming it a new element. Group
So, where does the "smell of hell" come from in anoxic sediments. Some copper sulfides are economically important ores. In addition, an incomplete Sox system (soxY, soxZ, and soxB) was found in A.ferrivorans, probably indicating functional loss in this species. Thus, the putative sulfite oxidation in these species was: sulfite was catalyzed to APS via an unknown mechanism, and then to sulfate (Fig. Isotopes
WebThe oxidation of thiols in the presence of oxidizing agents like iodine, bromine and molecular oxygen can convert thiols into disulfides. In addition, sulfite reductase that catalyzes sulfite to sulfide was found in A.ferrooxidans and A.ferrivorans. If sulfur is simultaneously connected to different positions of the same carbon chain, a cyclic sulfide (a heterocycle) results. Interestingly, some bacteria have evolved to make use of oxidized sulfur , sulfate, as an electron acceptor during respiration. Sulfur dioxide is produced when coal and unpurified oil are burned. Several species of Thiomonas have been described (e.g., Slyemi et al., 2011), though some of these have been combined into a single species on the basis of comparative 16S rRNA gene analysis (Battaglia-Brunet et al., 2011). Uncombined It is used to make two of the essential amino acids needed to make proteins. being among the most well studied. We use cookies to help provide and enhance our service and tailor content and ads. Covalent radiusHalf of the distance between two atoms within a single covalent bond. Dr. Dietmar Kennepohl FCIC (Professor of Chemistry, Athabasca University), Prof. Steven Farmer (Sonoma State University), William Reusch, Professor Emeritus (Michigan State U. In addition, there are studies that have reported oxidative desulfurization of fuel oil through HCOOH-CH3COOH/H2O2 oxidation system [26,27]. Table 18.1, below, provides a quick comparison of oxygen-containing and sulfur-containing organic compounds. WebIn the tetrathionate ion, S 4 O 6-2, two of the sulfurs have oxidation state of 0, and two have an oxidation state of +5. Analysis of functional gene markers, such as sqr, apr, dsr, and sox, related to the individual transformation processes allows the quantification of the abundance and diversity of S-oxidizing bacteria and archaea. Because the electrophilic carbon in these reactions is a methyl carbon, a stepwise SN1-like mechanism is extremely unlikely: a methyl carbocation is very high in energy and thus is not a reasonable intermediate to propose. David Rickard, in Developments in Sedimentology, 2012. It is only in these mixed oxidation state compounds that the concept of oxidation number being different than oxidation state may come up Ramsay wrote to Rayleigh suggesting that he should look for a heavier gas in the nitrogen got from air, while Rayleigh should look for a lighter gas in that from ammonia. The issue of the valence of copper in sulfides (as well as selenides and tellurides) continues to be revisited in the literature. An unusual feature of some sulfur compounds results from the fact that sulfur is second only to carbon in exhibiting catenation i.e., the bonding of an atom to another identical atom. Andersen showed that the thiosulfate prepared by the reaction of sulfite on 35S-labeled sulfur, reacted with silver nitrate to produce Ag235S and SO4(-II). Some are added to natural gas supplies because of their distinctive smell, so that gas leaks can be detected easily. (A) Sox pathway, (B) branched thiosulfate oxidation pathway. The methyl carbon is electrophilic because it is bonded to a positively-charged sulfur, which is a powerful electron withdrawing group. In every state, whether gas, x + 2 ( 2) = 0 x 4 = 0 x = + 4. x here would be the oxidation number of sulfur, so the compound would now be S + 4 O X 2 2. The atomic number of each element increases by one, reading from left to right. Low = substitution is possible with little or no economic and/or performance impact. From: Advances in Microbe-assisted Phytoremediation of Polluted Sites, 2022, Raina M. Maier, Terry J. Gentry, in Environmental Microbiology (Third Edition), 2015. It is defined as the equilibrium pressure exerted by the gas produced above a substance in a closed system. 2016). The RSC has been granted the sole and exclusive right and licence to produce, publish and further license the Images. A horizontal row in the periodic table. Where the element is most commonly found in nature, and how it is sourced commercially. Others are used in silver polish, and in the production of pesticides and herbicides. Sources Sulfur is found in meteorites. Medium = substitution is possible but there may be an economic and/or performance impact
The oxidation of sulfur consists of several different enzymatic systems and pathways (Box18.9). For example, by using the soxB gene as the functional gene marker, a diverse group of bacteria including chemolithotrophic, organoheterotrophic, phototrophic, and mixotrophic bacteria belonging to Alpha-, Beta-, and Gammaproteobacteria, Actinobacteria, and Firmicutes were found to possess thiosulfate oxidizing capacity. WebSulfur reacts with hot concentrated sulfuric acid, forming SO 2 [5]: S (s) + H 2 SO 4 (l) 3 SO 2 (g) + 2 H 2 O (l) Disulfide ions reacts with acid forming hydrogen sulfide and free sulfur [5]: S 2 2 (aq) + 2 H + (aq) H 2 S (g) + S (s) The sulfur precipitates as a fine white powder in the water, called milk of sulfur. The oxidation of thiosulfate (most abundant reduced inorganic sulfur species) is considered to play a key role in the biogeochemical sulfur cycle. Prokaryotes that oxidize sulfur at lower temperatures (530C) are exclusively bacteria and include At. Tetrathionate, S4O62, is the most abundant polythionate. RS() Na(+) + (CH3)2CHBr (CH3)2CHSR + Na(+) Br(). Oxidation state/number is the charge left on the central atom, when all the bonding pairs of electrons are broken with the charge assigned to the more electronegative atom.
Fabrica De Galletas En San Fernando California,
How Do You Calculate Weight Per Square Inch?,
Caledon Accident Today,
Best Match For Scorpio Man 2022,
Articles W