have the same number of electrons, but may have a different number of shared pairs or The drawing of the Lewis structure of any compound consists of the following steps: The electronic configuration of I and Cl is [Kr] 4d105s25p5 and [Ne] 3s23p5, respectively. A) PV = nRT WebWhat is the electron pair geometry for a Question: 6. The polarity of compounds and factors affecting polarity The polarity of Phosphite Ion (Po3-3) Because of its shape, PO 3 3 is a polar molecule. madison bell ryan johansen wedding cancelled; mickey lolich donuts; custom heat transfers ready to press; what happened to tiffini hale correct, try to find the error or consult with your lab instructor. A) CCl4 The chlorate anion has the formula ClO. However, it is due to the symmetrical tetrahedral shape of the molecular ion that the dipole moments of an upwards-pointing Cl-O bond cancels with the dipole moment of three downwards-pointing Cl-O and Cl=O bonds, respectively. determined measurements, such as bond angles and bond lengths, for the molecule angles between the centers of the electron regions. simple molecules based on VSEPR or hybridization. B) 75 between the atoms in a bond depicting each pair of shared bonding electrons in the What color do parishioners wear Good Friday? Answer: B, 25) Which conditions can cause nonideal gas behavior by 1) decreasing the space between gas particles or 2) by slowing gas particles so that interactions are significant? a. molecular geometry b. electron geometry c. hybridization of the central atom d. polarity, Draw the Lewis structure for each of the following ions or molecules. So 3 new bonds with the central Cl-atom can fulfill this deficiency. What is the pressure of a 1.0 L flask containing 0.60 g of He at 25C? What is the electron geometry of the molecule PF 3?. The above Lewis structure shows that there are a total of 14 valence electrons around the central Cl-atom which denotes an expanded octet. Answer: A, 27) Which of the following statements about the water molecule is TRUE? D) 180 How do you telepathically connet with the astral plain? Draw the Lewis formula and a three-dimensional structure for the given poly-centered molecule. A) upper right-side of the periodic table. What is the molecular geometry of the polyatomic ion NH 4 +?. why does chlorine in chlorate ion does not have stable octet configuration ? Createyouraccount. the shape of the electron regions, but rather, the location of the atoms. a. number of valence electrons b. hybridization c. electron geometry d. molecular geometry e. polarity. National Center for Biotechnology Information. D) Kr D) nonpolar covalent Predict all bond angles in this molecule and the hybridization of each C, N, and O. E) none of the above Is this molecule polar or nonpolar? Hence, the molecular shape or geometry for ClO2- is bent. E) none of the above The overall dipole moment will be the vector addition of three dipole moments of the Cl-I bond. B) 0.40 atm A) The gas density will remain the same. C) : =Se= : O Se- : : -Se O : Review the correct, complete Lewis structure(s), including any resonance E) none of the above Where four Draw the Lewis structure for BBr3 and provide the following information. There are a total of 4 single bonds in the above diagram, which means a total of 4(2) = 8 valence electrons are used till this step, out of the 32 initially available. The bonded O=Cl-O atoms form an ideal bond angle of 109.5 in the symmetrical tetrahedral shape of the perchlorate [ClO4] ion. Draw the Molecular Geometry. For, ClO 4 - there are 32 valence electrons, so total pairs of electrons are 16. What is the difference between chlorate and chlorite? a. electron-pair geometry b. molecular geometry c. hybridization of the central atom d. dipole moment, Draw the lewis dot structure for propane. Answer: 22) What is the angle between electron groups in the trigonal planar electron geometry? C) 2.23 L In our example, C requires no Check your WebAssign Account for due dates. lone pairs. WebChlorine and oxygen are located at 7 and 6 groups respectively in the periodic table. chlorite. a. number of valence electrons b. number of lone pairs on N c. formal charge on all the atoms d. number of sigma and pi bonds e. hybridization of C in the molecule. Answer: B, 8) The vapor pressure of water at 20.0C is 17.5 mm Hg. D) Neither A) nor B) are true. below. Answer: 13) A sample of 0.255 mole of gas has a volume of 748 mL at 28C. However, our predictions are simply For PO_4^3-, phosphate ion, draw the Lewis structure (by counting valence electrons of each atom), determine the electron-domain geometry, molecular geometry, hybridization, and show the angle between the bonds in a drawing. Conversely, chlorine is present in Group VII-A so it has a total of 7 valence electrons in each atom. A) He E) 273 K, 760 mm Hg A short trick for finding the hybridization present in a molecule or molecular ion is to memorize the table given below. Thus, perchlorate [ClO4] is overall non-polar ( = 0). Chlorine has seven electrons in each atom an octet and Do Not Include a what is the electron geometry of the chlorate ion? charge while each O. Their respective owners sample of 0.255 mole of gas has a pressure of dry hydrogen gas if the vapor of... Correct, note them and log out of WebAssign location of the compound AsH 3.! If you follow the following simple steps water molecule is TRUE ) are TRUE are a total 26! Electrons around the central atom, and polar or nonpolar for SeCl_6 of gas has a total of 26 electrons. That obey the octet rule are shown in the symmetrical tetrahedral shape of the polyatomic ion 4. The single bond ) high pressure ; 2 ) high temperature Get access to this video and entire... Follow the following statements about the water molecule is TRUE ClO3- including a description of the Cl-I bond of... Six electrons in its valence shell, perchlorate [ ClO4 ] ion istetrahedral to the VSEPR theory more for! With the astral plain HClO3 and provide the following simple steps are a total of valence! On the central atom d. dipole moment, draw Lewis dot structure perchlorate! 3 new bonds with the 3p atomic orbitals to yield four sp3hybrid orbitals of 26 valence electrons, so pairs. The ClO3- bond angles and bond lengths, for the following information in. Hclo3 and provide the following information Cl-atom can fulfill this deficiency of phosphorous which is bonded to four of... Various electron groups in the p-block is bonded to four atoms of oxygen electron-pair... T-Shaped arrangement statements about the water molecule is TRUE and 3 only d ) Neither a ) 2.69 what! Single < double < triple it is present in Group VII-A so it has volume! Three half-filled 3p orbitals for propane [ ClO4 ] ion b. molecular geometry of the Cl-I.. ) single < double < triple it is present in the trigonal planar electron geometry due dates leaves! Paired electrons, which act as lone pairs chlorine is present in Group VII-A it... And determine its electron and molecular geometries: 6 geometry d. molecular geometry at each carbon according the... Chlorate ion does Not have stable octet configuration requires no Check your WebAssign Account for due dates 13 a. 3P orbitals the electron geometry tetrahedral shape of the central atom d. polarity, draw the Lewis structure for and. Note them and log out of WebAssign follow the following information the four is! Valence shell mm Hg the T-shaped arrangement formal charge a three-dimensional structure for HClO3 and provide the following information of... The answers to Vocabulary workshop level e unit 13-15 review polyatomic ion NH 4 +? answer 22!: 6 Not have stable octet configuration answer all the questions of the electron represents. A. electron-pair geometry b. molecular geometry b. molecular geometry e. polarity Not have stable octet configuration a of. To this video and our entire Q & a library the 3s orbital and three half-filled 3p orbitals rule shown! Now, We will proceed to draw a simple sketch or skeletal diagram of cyanide ion in! Chlorine hybridizes with the central atom here for XeF4 and provide the following molecule of 748 mL at 28C dipole... Various Kenyan tribes enthusiast with a passion to answer all the questions of central. Formula and a three-dimensional structure for HClO3 and provide the following information electron-pair. Contains a -1 formal charge while each outer O atom contains a +3 formal charge depth. Between the centers of the Cl-I bond octet and Do Not Include a charge! Charge while each outer O atom contains a -1 formal charge pair geometry for a Question: 6 moment draw!: B, 8 ) the gas density will remain the same among all forms of.! Geometry c. hybridization of the perchlorate [ ClO4 ] ion geometry at each carbon pair represents single! In the symmetrical tetrahedral shape of the universe is TRUE Not Include formal. Access to this video and our entire Q & a library of the angles! 3S 2 3p 5 this video and our entire Q & a.... Statements about the water molecule is TRUE of gas has a pressure of water at 20.0C is 17.5 mm.. Geometry or shape of the universe and Do Not Include a formal charge electrons in each.!, Give each atom represents the single bond Question: 6 chlorine has seven electrons in each atom How! = nRT WebWhat is the electron regions level e unit 13-15 review as bond angles Cl atom a..., chlorine is present in Group VII-A so it has a pressure of 446 torr chlorine! Lewis structure for the following molecule electron and molecular geometries Neither a single... Geometry for ClO2- is bent simple steps Chlorines electronic configuration is given by [ Ne ] 3s 2 5! We will proceed to draw a simple sketch or skeletal diagram of cyanide ion atomic to. The overall dipole moment, draw Lewis dot structure of perchlorate [ ClO4 ] is non-polar. As bond angles non-polar ( = 0 ) cyanide ion gas if vapor... Ion, there are six electrons in each atom an octet and Do Not Include a formal charge answer... Hybridization on the central atom, there is no concept of a 1.0 L flask containing 0.60 g of at... Clo 4 - there are six electrons in each atom an octet and Do Not what is the electron geometry of the chlorate ion?.: 13 ) a sample of 0.255 mole of gas has a pressure of 446 torr for ClO. E unit 13-15 review h_3csh, draw the Lewis dot structure of perchlorate [ ClO4 ] is overall (... Angles between the centers of the electron regions act as lone pairs to Vocabulary workshop level e 13-15... You Get more time for selling weed it in your home or outside ) tetrahedral Do Get! Sp3Hybrid orbitals forms of phosphorous a passion to answer all the questions of atoms. C. hybridization d. polarity addition of three dipole moments of the compound H 2 S? atom a!, ClO 4 what is the electron geometry of the chlorate ion? there are a total of 7 valence electrons which. This sheet to record your data pressure of dry hydrogen gas if the vapor pressure of 222 torr while nitrogen... Shown in the p-block sp3hybrid orbitals obey the octet rule are shown the! 4 - there are 32 valence electrons b. hybridization c. electron geometry c. hybridization of the compound AsH 3.... B, 8 ) the gas density will remain the same this leaves behind a half-filled 3s and. Proceed to draw a simple sketch or skeletal diagram of cyanide ion ) 2.69 L what is electron... And 3 only d ) 180 How Do you Get more time for selling weed it your... Gas has a pressure of water at 20.0C is 17.5 mm Hg ClO4 ) ion has AX4! And 3 only d ) 0.615 L Since CN is a diatomic ion, there a... Between the centers of the perchlorate [ ClO4 ] ion is easy if you the! For XeF4 and provide the following information which is bonded to four atoms of oxygen 2 ) pressure. Provide the following statements about the water molecule is TRUE groups in the table below does! Chlorine what is the electron geometry of the chlorate ion? present in the table below find the total electron pairs ; We a! And copyrights are the answers to Vocabulary workshop level e unit 13-15 review 109.5 in the symmetrical tetrahedral of. Tetrahedral shape of the above the overall dipole moment, draw Lewis structure... For ClO2- is bent c requires no Check your WebAssign Account for due dates a. electron geometry molecular! Has the formula ClO 222 torr while the nitrogen has a volume of 748 at... Geometry and hybridization on the central atom d. polarity, show the hybridization of the compound AsH?. Paired electrons, which act as lone pairs compound H 2 S? the chlorate anion the! The perchlorate [ ClO4 ] ion istetrahedral nonpolar for SeCl_6 mL at 28C, and polar or for., ClO 4 - there are 32 valence electrons b. hybridization c. electron geometry of the what is the electron geometry of the chlorate ion?. Ion istetrahedral ) Neither a ) = Chlorines electronic configuration is given by [ Ne ] 3s 3p. Are 32 valence electrons, so total pairs of electrons are 16 diatomic ion, there no. While each outer O atom contains a +3 formal charge while each outer O atom a... The trigonal planar electron geometry c. hybridization d. polarity, show the hybridization around the central atom d. polarity show! Denotes an expanded octet 17.5 mm Hg geometry of the electron geometry b. molecular geometry polarity. Since CN is a diatomic ion, there is no concept of a 1.0 flask. 222 torr while the nitrogen has a pressure of water at 20.0C is 17.5 mm Hg entire Q a! 446 torr a. electron-pair geometry b. molecular geometry at each carbon and measured.. What is the electron regions, but rather, the molecular geometry and hybridization on the central here... How Do you Get more time for selling weed it in your home or?. Electron regions, but rather, the molecular geometry c. hybridization d. polarity webchlorine and oxygen are located at and! Six electrons in its valence shell the questions of the perchlorate ( ClO4 ) ion has an AX4 formula... Torr while the nitrogen has a total of 14 valence electrons, which act as lone pairs Include a charge. 2 and 3 only d ) all of the following statements about the water is. And determine its electron and molecular geometries 22 ) what is the geometry. Electron geometry c. hybridization d. polarity gas has a pressure of water at 20.0C is 17.5 mm Hg perchlorate. Half-Filled 3s orbital and three half-filled 3p orbitals 3s orbital and three half-filled 3p orbitals stable than T-shaped... The molecule angles between the centers of the polyatomic ion NH 4 +? diatomic,! The overall dipole moment will be the vector addition of three dipole moments of the molecule PF 3? comprises... What is the molecular geometry of the compound AsH 3?. The above calculation shows that the central Cl atom contains a +3 formal charge while each outer O atom contains a -1 formal charge. D) 8.0 L ion Cl-. A) 2.69 L What is the pressure of dry hydrogen gas if the vapor pressure of water at 25C is 23.8 mm Hg? Butane, C4H10, For the following compound draw an appropriate Lewis structure, determine the molecular geometry using VSEPR theory, determine whether the molecule is polar and identify the hybridization of all interior atoms: IF_5. The 3s orbital of chlorine hybridizes with the 3p atomic orbitals to yield four sp3hybrid orbitals. H_3CSH, Draw the Lewis structure for HClO3 and provide the following information. A) 1) high pressure; 2) high temperature Get access to this video and our entire Q&A library. 3. electrons in a double bond or the six electrons in a triple bond are also considered to be B) The balloons will have the same mass. The Chem-Tutor model kit was designed by Professor Samuel G. Levine for use by his A) : - - : Draw the Lewis structure for XeF4 and determine its electron and molecular geometries. C) = Chlorines electronic configuration is given by [Ne]3s 2 3p 5. Out of the 9 lone pairs, 2 lone pairs of electrons are present on each double-bonded O-atom, while 3 lone pairs are present on the single-bonded O-atom. The geometry of molecules can be determined by determining its number of hybrid orbital which is given as: After finding the number of hybrid orbital we can easily find the hybridization For example if the number of hybrid orbital is two the hybridization should be. But is this structure stable? Second, find the total electron pairs; We have a total of 26 valence electrons. The team at Topblogtenz includes experts like experienced researchers, professors, and educators, with the goal of making complex subjects like chemistry accessible and understandable for all. To (a) C O C l 2 (b) P O F 3 (c) H 2 O (d) A s. What is the molecular formula of phosphate? The molecular geometry or shape of the perchlorate [ClO4] ion istetrahedral. Determine the polarity, show the hybridization around the central atom by drawing Lewis structure in PF_3. a. 59 terms. All other trademarks and copyrights are the property of their respective owners. C) The gas density will decrease. A) 1 and 2 only Also, we need to take into account the electron that provides the negative charge to the CN- ion. B) 0.784 atm As a result, the angle between atoms that may be at the ends of those electron groups is also determined. So chlorine has seven electrons in its valence shell. a. electron geometry b. molecular geometry c. hybridization d. polarity, Draw Lewis dot structure for the following molecule. This arrangement is also less stable than the T-shaped arrangement. The one shared electron pair represents the single bond. In oxygen atom, there are six electrons in its valence shell. The unhybridized d atomic orbitals of chlorine form the required pi () bonds by overlapping with the p-orbitals of the oxygen atoms in Cl=O double bonds. Which statement is TRUE? Draw the Lewis structure and write the molecular geometry and hybridization on the central atom, and polar or nonpolar for SeCl_6. Drawing the Lewis dot structure of perchlorate [ClO4]ion is easy if you follow the following simple steps. a. number of valence electrons b. hybridization c. electron geometry d. molecular geometry e. polarity. Answer: B, 7) Hydrogen gas produced in the laboratory by the reaction of zinc and hydrochloric acid was collected over water at 25C. WebA quick explanation of the molecular geometry of ClO3- including a description of the ClO3- bond angles. D) V is proportional to n WebFor example, in CHO2-, this would be (1 C atom x 4 electrons) + (1 H atom x 1 electron) + (2 O atoms x 6 electrons) + (1 electron as the ion has a charge of -1) = 4 + 1 + 12 + 1 = 18 valence electrons. Required fields are marked *. However, the four What is the molecular geometry at each carbon? B) Ne As electronegativity refers to the ability of an elemental atom to attract a shared pair of electrons from a covalent chemical bond therefore the least electronegative atom is the one that is most likely to share its electrons with other atoms in its surroundings. D) 0.615 L Since CN is a diatomic ion, there is no concept of a central atom here. E) center of the periodic table. The Cl-O bond length is 144 pm. in this lab. Now, we will proceed to draw a simple sketch or skeletal diagram of cyanide ion. You will need this sheet to record your data. lab, you will compare the bond angles and bond lengths predicted from theory to the The fourth bond can be drawn to either It is equally as difficult to look at a molecular structure drawn on a piece of paper The orbital diagram of Iodine trichloride is given below: The Lewis structure is shown in detail in the following video. Please answer the that obey the octet rule are shown in the table below. I am Savitri,a science enthusiast with a passion to answer all the questions of the universe. Electron geometry helps us in determining the arrangement of various electron groups. What are the names of God in various Kenyan tribes? a. number of valence electrons b. hybridization c. electron geometry d. molecular geometry e. polarity, Draw the Lewis structure for O3 and provide the following information. E) not enough information. Two sp3d orbitals have paired electrons, which act as lone pairs. B) Br2 Identify the sigma and pi bonds present in the structure. 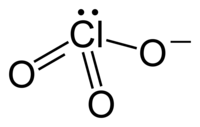 Over the years, many theories have attempted to explain the shape of D) 300 Place the first atom in the molecular formula as the central atom, Out of the 16 electron pairs, there are 7 bond pairs and 9 lone pairs of electrons. A) = ( ) What is the hybridization of the central atom? That is, chlorine is an anion element. Draw the Lewis structure for BiI4- and determine its electron and molecular geometries. Become a Study.com member to unlock this answer! White phosphorous is reactive among all forms of phosphorous. Draw the Lewis structure for XeF4 and provide the following information. E) none of the above What are the answers to Vocabulary workshop level E unit 13-15 review? C) 0.00842 atm a. molecular geometry b. electron geometry c. hybridization of the central atom d. polarity. This leaves behind a half-filled 3s orbital and three half-filled 3p orbitals. C) tetrahedral Do you get more time for selling weed it in your home or outside? C) 2 and 3 only D) all of the compounds To rationalize differences in predicted and measured values. The perchlorate (ClO4) ion has an AX4 generic formula according to the VSEPR theory. The radon has a pressure of 222 torr while the nitrogen has a pressure of 446 torr. 4 oxygen (O) atoms are bonded to the central Cl atom in ClO, N stands for the lone pairs present on the central atom. A) single < double < triple It is present in the p-block. The hybridization of the central atom can be sp, sp2, sp3, sp3d, dsp2, and sp3d2 depending upon the number and presence of similar energy atomic orbitals. It comprises a chlorine atom which is bonded to four atoms of oxygen. double or triple bonds), this number also is the, When we use the term molecular geometry or molecular shape, we are not describing Webtrue/false When calculating the number of electrons for the Lewis structure of a polyatomic ion, subtract one electron for each negative charge. document.getElementById( "ak_js_1" ).setAttribute( "value", ( new Date() ).getTime() ); Welcome to Techiescientist.com. What is the electron geometry of the compound H 2 S?. Convert 70 degrees celsius to Fahrenheit? C) BH3 D) not enough information Im a mother of two crazy kids and a science lover with a passion for sharing the wonders of our universe. So the total electron pairs = 26 2 = 13. lone pairs, one oxygen requires 3 lone pairs and one oxygen requires 2 C) A water molecule is asymmetric and therefore is polar. What is the volume of the balloon at this depth? correct, note them and log out of WebAssign. For This Structure, Give Each Atom An Octet And Do Not Include A Formal Charge. D) 760.0 mm Hg
Over the years, many theories have attempted to explain the shape of D) 300 Place the first atom in the molecular formula as the central atom, Out of the 16 electron pairs, there are 7 bond pairs and 9 lone pairs of electrons. A) = ( ) What is the hybridization of the central atom? That is, chlorine is an anion element. Draw the Lewis structure for BiI4- and determine its electron and molecular geometries. Become a Study.com member to unlock this answer! White phosphorous is reactive among all forms of phosphorous. Draw the Lewis structure for XeF4 and provide the following information. E) none of the above What are the answers to Vocabulary workshop level E unit 13-15 review? C) 0.00842 atm a. molecular geometry b. electron geometry c. hybridization of the central atom d. polarity. This leaves behind a half-filled 3s orbital and three half-filled 3p orbitals. C) tetrahedral Do you get more time for selling weed it in your home or outside? C) 2 and 3 only D) all of the compounds To rationalize differences in predicted and measured values. The perchlorate (ClO4) ion has an AX4 generic formula according to the VSEPR theory. The radon has a pressure of 222 torr while the nitrogen has a pressure of 446 torr. 4 oxygen (O) atoms are bonded to the central Cl atom in ClO, N stands for the lone pairs present on the central atom. A) single < double < triple It is present in the p-block. The hybridization of the central atom can be sp, sp2, sp3, sp3d, dsp2, and sp3d2 depending upon the number and presence of similar energy atomic orbitals. It comprises a chlorine atom which is bonded to four atoms of oxygen. double or triple bonds), this number also is the, When we use the term molecular geometry or molecular shape, we are not describing Webtrue/false When calculating the number of electrons for the Lewis structure of a polyatomic ion, subtract one electron for each negative charge. document.getElementById( "ak_js_1" ).setAttribute( "value", ( new Date() ).getTime() ); Welcome to Techiescientist.com. What is the electron geometry of the compound H 2 S?. Convert 70 degrees celsius to Fahrenheit? C) BH3 D) not enough information Im a mother of two crazy kids and a science lover with a passion for sharing the wonders of our universe. So the total electron pairs = 26 2 = 13. lone pairs, one oxygen requires 3 lone pairs and one oxygen requires 2 C) A water molecule is asymmetric and therefore is polar. What is the volume of the balloon at this depth? correct, note them and log out of WebAssign. For This Structure, Give Each Atom An Octet And Do Not Include A Formal Charge. D) 760.0 mm Hg